Chemical composition, antioxidant activities, and enzyme inhibitory effects of Lespedeza bicolour Turcz. essential oil
- PMID: 39912419
- PMCID: PMC11803819
- DOI: 10.1080/14756366.2025.2460053
Chemical composition, antioxidant activities, and enzyme inhibitory effects of Lespedeza bicolour Turcz. essential oil
Abstract
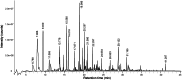
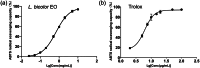
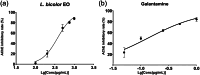
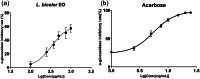
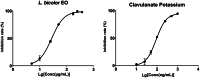
Lespedeza bicolour Turcz. is a traditional medicinal plant with a wide range of ethnomedicinal values. The main components of L. bicolour essential oil (EO) were β-pinene (15.41%), β-phellandrene (12.43%), and caryophyllene (7.79%). The EO of L. bicolour showed antioxidant activity against ABTS radical and DPPH radical with an IC50 value of 0.69 ± 0.03 mg/mL and 10.44 ± 2.09 mg/mL, respectively. The FRAP antioxidant value was 81.96 ± 6.17 μmol/g. The EO had activities against acetylcholinesterase, α-glucosidase, and β-lactamase with IC50 values of 309.30 ± 11.16 μg/mL, 360.47 ± 35.67 μg/mL, and 27.54 ± 1.21 μg/mL, respectively. Molecular docking showed methyl dehydroabietate docked well with all tested enzymes. Sclareol and (+)-borneol acetate showed the strongest binding affinity to α-glucosidase and β-lactamase, respectively. The present study provides a direction for searching enzyme inhibitors for three tested enzymes and shows L. bicolour EO possesses the potential to treat a series of diseases.
Keywords: Lespedeza bicolour Turcz.; bioactivities; chemical composition; essential oil; molecular docking.
Conflict of interest statement
There are no competing interests to declare.
Figures

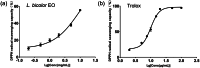



Similar articles
-
Chemical Composition, Antioxidant, and Enzyme Inhibitory Activities of Artemisia schmidtiana Maxim. Essential Oil.Biomolecules. 2025 May 19;15(5):736. doi: 10.3390/biom15050736. Biomolecules. 2025. PMID: 40427629 Free PMC article.
-
Antidiabetic and Antioxidant Activities of Indian Bay Leaf (Cinnamomum tamala (Buch.-Ham.) T. Nees & Eberm.) Essential Oils Collected from Meghalaya.Chem Biodivers. 2024 Nov;21(11):e202400879. doi: 10.1002/cbdv.202400879. Epub 2024 Sep 16. Chem Biodivers. 2024. PMID: 39075867
-
Chemical Composition and Pharmacological Evaluation and of Toddalia asiatica (Rutaceae) Extracts and Essential Oil by in Vitro and in Silico Approaches.Chem Biodivers. 2021 Apr;18(4):e2000999. doi: 10.1002/cbdv.202000999. Epub 2021 Mar 19. Chem Biodivers. 2021. PMID: 33738900
-
GC/MS Profiling, In Vitro and In Silico Pharmacological Screening and Principal Component Analysis of Essential Oils from Three Exotic and Two Endemic Plants from Mauritius.Chem Biodivers. 2021 Mar;18(3):e2000921. doi: 10.1002/cbdv.202000921. Epub 2021 Feb 17. Chem Biodivers. 2021. PMID: 33594799
-
Essential Oil From Tetrapanax papyrifer (Hook.) K. Koch: Chemical Composition, Antioxidant Activity, α-Glucosidase Inhibitory Effect Integrating Molecular Docking Analysis.Chem Biodivers. 2025 May;22(5):e202402533. doi: 10.1002/cbdv.202402533. Epub 2024 Dec 31. Chem Biodivers. 2025. PMID: 39714382
Cited by
-
Optimized Extraction, Comprehensive Chemical Profiling, and Antioxidant Evaluation of Volatile Oils from Wurfbainia villosa (Lour.) Škorničk. & A.D.Poulsen Leaves.Plants (Basel). 2025 Jul 3;14(13):2041. doi: 10.3390/plants14132041. Plants (Basel). 2025. PMID: 40648050 Free PMC article.
-
Structural Bioinformatics Applied to Acetylcholinesterase Enzyme Inhibition.Int J Mol Sci. 2025 Apr 17;26(8):3781. doi: 10.3390/ijms26083781. Int J Mol Sci. 2025. PMID: 40332446 Free PMC article. Review.
-
Comparative Analysis of Lespedeza Species: Traditional Uses and Biological Activity of the Fabaceae Family.Molecules. 2025 Apr 30;30(9):2013. doi: 10.3390/molecules30092013. Molecules. 2025. PMID: 40363818 Free PMC article. Review.
-
Antimicrobial, Antioxidant, and α-Glucosidase-Inhibitory Activities of Prenylated p-Hydroxybenzoic Acid Derivatives from Oberonia ensiformis.Molecules. 2025 May 12;30(10):2132. doi: 10.3390/molecules30102132. Molecules. 2025. PMID: 40430304 Free PMC article.
-
Chemical Composition, Antioxidant, and Enzyme Inhibitory Activities of Artemisia schmidtiana Maxim. Essential Oil.Biomolecules. 2025 May 19;15(5):736. doi: 10.3390/biom15050736. Biomolecules. 2025. PMID: 40427629 Free PMC article.
References
-
- Zhu Y, Wang K, Jia X, Fu C, Yu H, Wang Y.. Antioxidant peptides, the guardian of life from oxidative stress. Med Res Rev. 2024;44(1):275–364. - PubMed
-
- Sies H, Jones DP.. Reactive oxygen species (ROS) as pleiotro pic physiological signaling agents. Nat Rev Mol Cell Biol. 2020;21(7):363–383. - PubMed
-
- Praticò D. Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal. Trends Pharmacol Sci. 2008;29(12):609–615. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
