Aging-Associated Liver Sinusoidal Endothelial Cells Dysfunction Aggravates the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease
- PMID: 39912563
- PMCID: PMC12073894
- DOI: 10.1111/acel.14502
Aging-Associated Liver Sinusoidal Endothelial Cells Dysfunction Aggravates the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease
Abstract
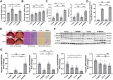
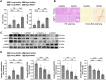
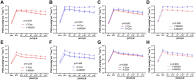
Aging increases the susceptibility to metabolic dysfunction-associated steatotic liver disease (MASLD). Liver sinusoidal endothelial cells (LSECs) help in maintaining hepatic homeostasis, but the contribution of age-associated LSECs dysfunction to MASLD is not clear. The aim of this study was to investigate the effect of aging-associated LSECs dysfunction on MASLD. Free fatty acid-treated AML12 cells were co-cultured with young and etoposide-induced senescent TSEC cells to evaluate the senescence-associated endothelial effects on the lipid accumulation in hepatocytes. In addition, young and aged rats were subjected to methionine-choline-deficient diet-induced metabolic dysfunction-associated steatohepatitis (MASH). Hepatic hemodynamics and endothelial dysfunction were evaluated by in situ liver perfusion. Liver tissue samples from young and aged healthy controls and MASH patients were also analyzed. Steatotic AML12 cells co-cultured with young TSEC cells showed less lipid accumulation, and such effect was abolished by eNOS inhibitor or with senescent TSEC cells. However, co-culture with resveratrol-treated senescent TSEC cells could partially resume the NO-mediated protective effects of endothelial cells. Furthermore, aged MASH rats showed more severe liver injury, steatosis, fibrosis, and endothelial and microcirculatory dysfunction. In addition, aged MASH patients showed more pronounced liver injury and fibrosis with lower hepatic eNOS, p-eNOS, and SIRT1 protein levels than in young patients. Senescence compromises the protective effects of LSECs against hepatocyte steatosis. In addition, aging aggravates not only liver steatosis and fibrosis but also intensifies LSECs dysfunction in MASH rats. Accordingly aged MASH patients also showed endothelial dysfunction with more severe liver injury and fibrosis.
Keywords: aging; fibrosis; liver sinusoidal endothelial cells; metabolic dysfunction–associated steatotic liver disease.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Cordero‐Herrera, I. , Kozyra M., Zhuge Z., et al. 2019. “AMP‐Activated Protein Kinase Activation and NADPH Oxidase Inhibition by Inorganic Nitrate and Nitrite Prevent Liver Steatosis.” Proceedings of the National Academy of Sciences of the United States of America 116, no. 1: 217–226. 10.1073/pnas.1809406115. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

