Comparative assessment of a restored and natural wetland using 13C-DNA SIP reveals a higher potential for methane production in the restored wetland
- PMID: 39912641
- PMCID: PMC11921397
- DOI: 10.1128/aem.02161-24
Comparative assessment of a restored and natural wetland using 13C-DNA SIP reveals a higher potential for methane production in the restored wetland
Abstract
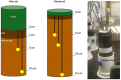
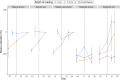
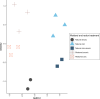
Wetlands are the largest natural source of methane (CH4), a potent greenhouse gas produced by methanogens. Methanogenesis rates are controlled by environmental factors such as redox potential, temperature, and carbon and electron acceptor availability and are presumably dependent on the composition of the active methanogen community. We collected intact soil cores from a restored and natural freshwater depressional wetland on Maryland's Delmarva Peninsula (USA) to assess the effects of wetland restoration and redox shifts on microbial processes. Intact soil cores were incubated under either saturated (anoxic) or unsaturated (oxic) conditions and amended with 13C-acetate for quantitative stable isotope probing (qSIP) of the 16S rRNA gene. Restored wetland cores supported a distinct community of methanogens compared to natural cores, and acetoclastic methanogens putatively identified in the genus Methanosarcina were among the most abundant taxa in restored anoxic and oxic cores. The active microbial communities in the restored wetland cores were also distinguished by the unique presence of facultatively anaerobic bacteria belonging to the orders Firmicutes and Bacteroidetes. In natural wetland incubations, methanogen populations were not among the most abundant taxa, and these communities were instead distinguished by the unique presence of aerobic bacteria in the phyla Acidobacteria, Actinobacteria, and class Alphaproteobacteria. Iron-reducing bacteria, in the genus Geobacter, were active across all redox conditions in both the restored and the natural cores, except the natural oxic-anoxic condition. These findings suggest an overall higher potential for methanogenesis in the restored wetland site compared to the natural wetland site, even when there is evidence of Fe reduction.IMPORTANCEMethane (CH4) is a potent greenhouse gas with an atmospheric half-life of ~10 years. Wetlands are the largest natural emitters of CH4, but CH4 dynamics are difficult to constrain due to high spatial and temporal variability. In the past, wetlands were drained for agriculture. Now, restoration is an important strategy to increase these ecosystems' potential for sequestering carbon. However, the consequences of wetland restoration on carbon biogeochemistry are under-evaluated, and a thorough assessment of the active microbial community as a driver of biogeochemical changes is needed. Particularly, the effects of seasonal flooding/drying cycles in geographically isolated wetlands might have implications for CH4 emissions in both natural and restored wetlands. Here, we found that active microbial communities in natural and restored wetlands responded differently to flooding and drying regimes, resulting in differences in CH4 production potentials. Restored wetlands had a higher potential for CH4 production compared to natural wetlands. Our results show that controls on CH4 production in a restored wetland are complex, and dynamics of active microbial communities are linked to seasonal dry-wet cycles.
Keywords: methanogenesis; stable isotope probing; wetland soil microbial ecology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Saunois M, Stavert AR, Poulter B, Bousquet P, Canadell JG, Jackson RB, Raymond PA, Dlugokencky EJ, Houweling S, Patra PK, et al. . 2020. The global methane budget 2000–2017. Earth Syst Sci Data 12:1561–1623. doi:10.5194/essd-12-1561-2020 - DOI
-
- Mitsch WJ, Bernal B, Nahlik AM, Mander Ü, Zhang L, Anderson CJ, Jørgensen SE, Brix H. 2013. Wetlands, carbon, and climate change. Landscape Ecol 28:583–597. doi:10.1007/s10980-012-9758-8 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

