Novel mechanistic insights of the potential role of gasotransmitters and autophagy in the protective effect of metformin against hepatic ischemia/reperfusion injury in rats
- PMID: 39912902
- PMCID: PMC12263796
- DOI: 10.1007/s00210-025-03837-1
Novel mechanistic insights of the potential role of gasotransmitters and autophagy in the protective effect of metformin against hepatic ischemia/reperfusion injury in rats
Abstract
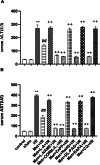
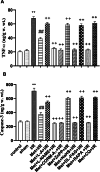
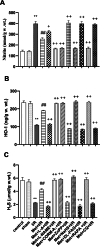
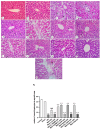
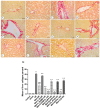
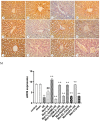
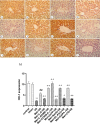
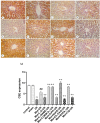
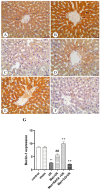
Metformin exerts antidiabetic and pleiotropic effects. This study investigated the function and mechanisms of gasotransmitters and autophagy in the metformin-induced protection against ischemia/reperfusion injury (I/RI). According to measurements of serum hepatic function indicators and histopathological evaluation, metformin protected against hepatic I/RI-induced impairment of liver function and structure. In addition, metformin inhibited hepatic I/RI-induced hepatic oxidative stress, nitrosative stress, inflammation, and apoptosis. Also, it suppressed hepatic I/RI-induced decrease in hepatic heme oxygenase-1 (HO-1) and hydrogen sulfide (H2S) levels and increase in nitric oxide (NO) production. Furthermore, metformin inhibited hepatic I/RI-induced decrease in protein expressions of endothelial NO synthase (eNOS), HO-1, cystathionine γ-lyase (CSE), and Beclin-1 and increase in the protein expression of inducible NO synthase (iNOS) in the liver tissue. Co-administration of the NO biosynthesis inhibitor, L-NAME, carbon monoxide(CO)-releasing molecule-A1 (CORM-A1), the H2S donor, NaHS, or the autophagy stimulator, rapamycin (RAPA), enhanced all effects of metformin. The NO donor, L-arginine, the CO biosynthesis inhibitor, zinc protoporphyrin, the H2S biosynthesis inhibitor, DL-propargylglycine, or the autophagy inhibitor, chloroquine (CQ), antagonized the effects of metformin. These findings reveal, for the first time, that increasing CO, H2S, and autophagy levels with subsequent decreasing NO level play a critical role in metformin's protective action against hepatic I/RI. The ability of L-NAME, CORM-A1, NaHS, and RAPA to boost metformin's protective effect in hepatic I/RI may positively be attributed to their ability to lower hepatic oxidative stress, nitrosative stress, inflammation, and apoptosis.
Keywords: Autophagy; CO; H2S; Hepatic ischemia/reperfusion injury (I/RI); Metformin; NO.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The experiments reported here were approved by our Institutional Ethics Committee (ethical approval No. 17300541, 2020). Conflict of interest: The authors declare no competing interests.
Figures


References
-
- Abdelsameea AA, Abbas NA, Abdel Raouf SM (2017) Liraglutide attenuates partial warm ischemia-reperfusion injury in rat livers. Naunyn Schmiedebergs Arch Pharmacol 390:311–19 - PubMed
-
- Abdel-Zaher AO, Abd-Ellatief RB, Aboulhagag NA, Farghaly HSM, Al-Wasei FMM (2021) The potential relationship between gasotransmitters and oxidative stress, inflammation and apoptosis in lead-induced hepatotoxicity in rats. Tissue Cell 71:101511 - PubMed
-
- Abe T, Yazawa K, Fujino M, Imamura R, Hatayama N, Kakuta Y, Tsutahara K, Okumi M, Ichimaru N, Kaimori JY, Isaka Y, Seki K, Takahara S, Li XK, Nonomura N (2017) High-pressure carbon monoxide preserves rat kidney grafts from apoptosis and inflammation. Lab Invest 97:468–77 - PubMed
-
- Adach W, Olas B (2019) Carbon monoxide and its donors - their implications for medicine. Future Med Chem 11:61–73 - PubMed
-
- Afshari H, Noori S, Zarghi A (2023) A novel combination of metformin and resveratrol alleviates hepatic steatosis by activating autophagy through the cAMP/AMPK/SIRT1 signaling pathway. Naunyn Schmiedebergs Arch Pharmacol 396:3135–48 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

