Piano level laser therapy versus epidermal growth factor injection for painful myogenic temporomandibular disorder (a randomized clinical trial)
- PMID: 39912963
- PMCID: PMC11802707
- DOI: 10.1007/s00784-025-06189-5
Piano level laser therapy versus epidermal growth factor injection for painful myogenic temporomandibular disorder (a randomized clinical trial)
Abstract
Objective: The aim of this clinical trial was to evaluate the effectiveness of Piano level laser therapy using Nd-YAG laser and intramuscular EGF injection in pain alleviation, function, and quality of life improvement in patients suffering from myogenic TMD.
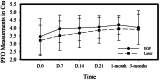
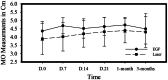
Materials and methods: A randomized clinical trial was performed on 29 patients suffering from chronic painful myogenic TMD based on diagnostic criteria for temporomandibular disorders. Group I (n = 13patients) was treated using 1064 nm Nd-YAG Laser (4 sessions once/week). Group II (n = 14 patients) was treated by intramuscular injection of EGF. Pain using numerical rating score, pain free opening and unassisted maximum opening were measured at baseline, 7,14,21 days, 1 and 3 months. Quality of life using OHIP-14 was assessed at baseline, 1 and 3 months.
Results: Results showed that there was a significant pain reduction (P < 0.000) and increase in pain free opening (P < 0.0001) in both test groups. However, only group I showed a significant increase in maximum opening (P = 0.007). Quality of life significantly improved in both groups (P = 0.0001). There was no significant difference between the two treatments in pain scores, pain free opening, maximum opening nor quality of life.
Conclusion: Both treatment modalities offered effective and cost-effective non- to minimally invasive treatment options for myogenic TMD with no side effects.
Clinical relevance: Myogenic TMD forms a public health issue and is a common musculoskeletal problem causing pain and disability. The proposal of effective, non-invasive, and affordable treatment options can help solve this issue.
Keywords: HILT; Injection; Non-invasive; Photobiomodulation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This clinical trial received the approval of the ethical committee of Faculty of Dentistry, Alexandria University, Egypt in December 2022. This clinical trial was registered at ClinicalTrials.gov Identifier: NCT06044974. Consent to participate: Participants of this clinical trial were treated according to the principles of the modified Helsinki’s code for human clinical studies [26]. Informed consent: An informed written consent was obtained from each patient before the start of the clinical procedures. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Effect of Nd-YAG Laser Versus Epidermal Growth Factor Injection on Salivary Pain Mediators in Myogenic Temporomandibular Disorders (A Randomized Clinical Trial).J Oral Pathol Med. 2025 Sep 2. doi: 10.1111/jop.70053. Online ahead of print. J Oral Pathol Med. 2025. PMID: 40891771
-
Psychological therapies for temporomandibular disorders (TMDs).Cochrane Database Syst Rev. 2022 Aug 11;8(8):CD013515. doi: 10.1002/14651858.CD013515.pub2. Cochrane Database Syst Rev. 2022. PMID: 35951347 Free PMC article.
-
Yoga for chronic non-specific low back pain.Cochrane Database Syst Rev. 2022 Nov 18;11(11):CD010671. doi: 10.1002/14651858.CD010671.pub3. Cochrane Database Syst Rev. 2022. PMID: 36398843 Free PMC article.
-
Injected corticosteroids for treating plantar heel pain in adults.Cochrane Database Syst Rev. 2017 Jun 11;6(6):CD009348. doi: 10.1002/14651858.CD009348.pub2. Cochrane Database Syst Rev. 2017. PMID: 28602048 Free PMC article.
-
The Comparison of the Efficacy and Safety of Fractional 1064 nm Nd:YAG Picosecond Laser and Fractional Q-Switched 1064-nm Nd:YAG Laser in the Improvement of Photoaging: A Split-Face Study.J Cosmet Dermatol. 2025 Jul;24(7):e70307. doi: 10.1111/jocd.70307. J Cosmet Dermatol. 2025. PMID: 40589289 Free PMC article.
Cited by
-
Advances in orofacial pain research: a bibliometric analysis.Front Neurol. 2025 Aug 14;16:1609437. doi: 10.3389/fneur.2025.1609437. eCollection 2025. Front Neurol. 2025. PMID: 40895114 Free PMC article.
References
-
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet J-P, List T, Svensson P, Gonzalez Y, Lobbezoo F (2014) Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J oral Facial pain Headache 28(1):6 - PMC - PubMed
-
- Ananthan S, Benoliel R (2020) Chronic orofacial pain. J Neural Transm 127(4):575–588 - PubMed
-
- Valentino R, Cioffi I, Vollaro S, Cimino R, Baiano R, Michelotti A (2019) Jaw muscle activity patterns in women with chronic TMD myalgia during standardized clenching and chewing tasks. CRANIO® - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

