The role of electroencephalography in epilepsy research-From seizures to interictal activity and comorbidities
- PMID: 39913107
- PMCID: PMC12097480
- DOI: 10.1111/epi.18282
The role of electroencephalography in epilepsy research-From seizures to interictal activity and comorbidities
Abstract
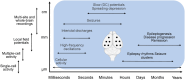
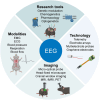
Electroencephalography (EEG) has been instrumental in epilepsy research for the past century, both for basic and translational studies. Its contributions have advanced our understanding of epilepsy, shedding light on the pathophysiology and functional organization of epileptic networks, and the mechanisms underlying seizures. Here we re-examine the historical significance, ongoing relevance, and future trajectories of EEG in epilepsy research. We describe traditional approaches to record brain electrical activity and discuss novel cutting-edge, large-scale techniques using micro-electrode arrays. Contemporary EEG studies explore brain potentials beyond the traditional Berger frequencies to uncover underexplored mechanisms operating at ultra-slow and high frequencies, which have proven valuable in understanding the principles of ictogenesis, epileptogenesis, and endogenous epileptogenicity. Integrating EEG with modern techniques such as optogenetics, chemogenetics, and imaging provides a more comprehensive understanding of epilepsy. EEG has become an integral element in a powerful suite of tools for capturing epileptic network dynamics across various temporal and spatial scales, ranging from rapid pathological synchronization to the long-term processes of epileptogenesis or seizure cycles. Advancements in EEG recording techniques parallel the application of sophisticated mathematical analyses and algorithms, significantly augmenting the information yield of EEG recordings. Beyond seizures and interictal activity, EEG has been instrumental in elucidating the mechanisms underlying epilepsy-related cognitive deficits and other comorbidities. Although EEG remains a cornerstone in epilepsy research, persistent challenges such as limited spatial resolution, artifacts, and the difficulty of long-term recording highlight the ongoing need for refinement. Despite these challenges, EEG continues to be a fundamental research tool, playing a central role in unraveling disease mechanisms and drug discovery.
Keywords: EEG; analysis; animal models; genetic epilepsies; high‐frequency oscillations; mechanisms; preclinical.
© 2025 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
C.P.L., M.O.C., M.dC., M.M., S.B., C.R.R., and P.J. have no conflicts of interest related to the manuscript topic. A.S.G. is the Editor‐in‐Chief of
Figures

References
-
- Cybulski N, Jelenska‐Macieszyna X. Action currents of the cerebral cortex Bull. Acad Sci Cracov. 1914;B:776–781.
-
- Penfield W, Jasper HH. Epilepsy and the functional anatomy of the human brain 1st ed. Boston: Little; 1954.
-
- Aladjalova NA. Infra‐slow rhythmic oscillations of the steady potential of the cerebral cortex. Nature. 1957;179(4567):957–959. - PubMed
-
- Bureš J, Petráň M, Zachar J. Electrophysiological methods in biological research. 2d ed. Praha, New York: House of the Czechoslovak Academy of Sciences; Academic Press; 1962.
Publication types
MeSH terms
Grants and funding
- 20/FFP-P/8613/SFI_/Science Foundation Ireland/Ireland
- MR/X004317/1/MRC_/Medical Research Council/United Kingdom
- R01 NS127524/NS/NINDS NIH HHS/United States
- ERDF-ProjectBraindynamicsCZ.02.01.01/00/22_008/0004643/Ministerstvo školství, mládeže a tělovýchovy
- U54 NS100064/NS/NINDS NIH HHS/United States
- LCF/PR/HR21/52410030/Fundación La Caixa
- W81XWH-22-1-0510/U.S. Department of Defense
- 16/RC/3948/SFI_/Science Foundation Ireland/Ireland
- LCF/PR/HR22/52420005/Fundación La Caixa
- 21-17564S/Grantová agentura České republiky
- NU21-08-00533/Agentura pro zdravotnický výzkum České republiky
- R01NS127524/NH/NIH HHS/United States
- R21AG086880/AG/NIA NIH HHS/United States
- R21 AG086880/AG/NIA NIH HHS/United States
- UNCE24/MED/021/Univerzita Karlova
- LX22NPO5107/Ministerstvo školství, mládeže a tělovýchovy
- W81XWH-22-1-0210/U.S. Department of Defense
- U54 NS100064/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

