Pembrolizumab in Patients With Advanced Clear Cell Gynecological Cancer: A Phase 2 Nonrandomized Clinical Trial
- PMID: 39913118
- PMCID: PMC11803509
- DOI: 10.1001/jamaoncol.2024.6797
Pembrolizumab in Patients With Advanced Clear Cell Gynecological Cancer: A Phase 2 Nonrandomized Clinical Trial
Abstract
Importance: Advanced clear cell gynecological cancers (CCGCs) have a poor prognosis, with response rates to second-line chemotherapy less than 8%. Preliminary clinical activity with programmed cell death 1 protein (PD-1) inhibitors reported in CCGC merits further investigation.
Objective: To assess the clinical benefit of pembrolizumab in patients with previously treated advanced CCGC.
Design, setting, and participants: The PEACOCC trial is a single-arm multicenter phase 2 trial conducted at 5 UK centers investigating the clinical benefit and safety of pembrolizumab. PD-1 inhibitor-naive patients with histologically confirmed advanced CCGC, radiological disease progression following 1 or more prior courses of chemotherapy, and an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0 to 1 were included. Patients were enrolled from March 2019 to October 2021, with data collected until July 2024.
Interventions: Pembrolizumab, 200 mg, intravenously every 21 days up to 2 years until progression, discontinuation due to toxic effects, or patient/clinician decision. Up to 1 year of retreatment on diseases progression, if stable disease, partial response, or complete response at 2 years.
Main outcomes and measures: The primary end point was progression-free survival (PFS) rate at 12 weeks using Response Evaluation Criteria in Solid Tumors version 1.1 to detect a 12-week PFS rate of 33% or greater and exclude a PFS rate of less than 15%, with 90% power and 1-sided 5% significance level. Secondary end points included objective response rate, duration of response, PFS, overall survival, safety, and quality of life.
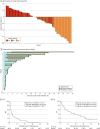
Results: A total of 48 patients were eligible. The median (range) age was 58.5 (32-77) years, and 26 (54%) had an ECOG PS score of 0 and 22 (46%) had an ECOG PS score of 1; 41 (85%) had ovarian, 6 (13%) had endometrial, and 1 (2%) had cervical advanced CCGC. The median (range) courses prior therapy was 3 (1-6); 19 patients (40%) received prior anti-angiogenic therapy, and 19 (40%) had a platinum-free interval of more than 12 months. Grade 3 treatment-related adverse events were observed in 9 patients (19%), and no patients had grade 4 or 5 adverse events. A total of 45 of 46 patients (98%) had mismatch repair-proficient tumors. The 12-week PFS rate was 42% (95% CI, 28-57), and the best objective response rate was 25% (95% CI, 14-40), with 12 partial responses. After a median follow-up of 46.9 months (95% CI, 43.4-55.0), the median PFS was 2.7 months (95% CI, 1.3-5.4), and the median overall survival was 14.8 months (95% CI, 6.7-28.2).
Conclusions and relevance: The PEACOCC trial showed clinical benefit with pembrolizumab in patients with previously treated advanced CCGC, of whom all except 1 had MMR-proficient disease. Clinical outcomes were durable with an overall tolerable safety profile, justifying further evaluation of pembrolizumab monotherapy for advanced CCGC in a randomized clinical trial.
Trial registration: ClinicalTrials.gov Identifier: NCT03425565.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

