FOLFIRINOX-3 plus bevacizumab (bFOLFIRINOX3) in chemo-refractory metastatic colorectal cancer: a multicenter phase II trial
- PMID: 39913183
- PMCID: PMC11881852
- DOI: 10.1080/14796694.2025.2461446
FOLFIRINOX-3 plus bevacizumab (bFOLFIRINOX3) in chemo-refractory metastatic colorectal cancer: a multicenter phase II trial
Abstract
Purpose: A phase I study of FOLFIRINOX3-bevacizumab (bFOLFIRINOX3)defined the RP2D for irinotecan at 70 mg/m² and showed promising activity. This phase II trial aimed to evaluate the efficacy of bFOLFIRINOX-3 in chemorefractory metastatic colorectal cancer (mCRC).
Methods: In phase II, chemorefractory mCRC were enrolled. The regimen tested consisted of bevacizumab (5 mg/kg), folinic acid(400 mg/m²), 5-fluorouracil (2400 mg/m² for 46 h), oxaliplatin (85 mg/m²) and irinotecan (70 mg/m² administered before and after infusion of 5-fluorouracil). The primary endpoint was efficacy defined by 2-month progression-free survival(PFS). Secondary endpoints included objective response, median PFS, overall survival (OS) and toxicity.
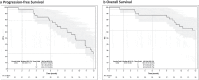
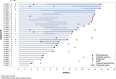
Results: 32 patients were enrolled (October 2018 to December 2022); median age 62.5 years (range 32-78). The majority had been treated with several previous lines of chemotherapy (median 3, range [1-8]). Median follow up was 12 months (range [1.5-12]). Two-month PFS was 96.9%. Best objective response rate (ORR) was 28.1%. Median PFS was 9.4 months (95%CI [6.9;11.5]) and median OS was not reached (95% [11.6; NR]). Grade 3 adverse events occurred in 81.2%; mostly diarrhea (37.5%) and neutropenia (12.5%). Grade 3 diarrhea consistently resolved after irinotecan dose reduction. The most common drug-related adverse events (all grades) were diarrhea (96.9%), fatigue (68.8%), nausea (68.7%), anemia (56.3%), peripheral neuropathy (53.4%) and thrombopenia (40.6%).
Conclusion: The combination of bFOLFIRINOX-3 yielded 2-month PFS of 96.9% and best ORR of 28.1%, and was well tolerated. These results are promising in chemotherapy refractory mCRC and provide a rationale for future randomized phase III trials.
Clinical trial registration: NCT03795311 (clinicaltrials.gov).
Keywords: Colorectal cancer; chemotherapy; irinotecan; metastasis; survival.
Plain language summary
This study looked at a new treatment option for patients with advanced colorectal cancer that no longer responds to standard chemotherapy. The treatment combines several cancer drugs: bevacizumab (which blocks blood vessel growth in tumors) and a chemotherapy regimen called FOLFIRINOX-3 (a mix of four drugs). Researchers aimed to see if this combination, called bFOLFIRINOX-3, could slow down cancer progression and how well patients could tolerate it.A total of 32 patients participated in the study. Most had already undergone multiple treatments for their cancer. The results were encouraging: 97% of patients had no worsening of their cancer after two months of treatment. Additionally, about 28% of patients saw a reduction in the size of their tumors. On average, patients went about 9 months before their cancer started to grow again, and the average overall survival time was not yet reached by the end of the study, indicating a potentially significant benefit.Side effects were common but manageable. Severe diarrhea was the most frequent issue, affecting 38% of patients, but it improved with a lower dose of one of the drugs. Other side effects included fatigue, nausea, low blood counts, and numbness in the hands and feet.Overall, this treatment showed promise for people with advanced colorectal cancer who have few other options. Further research in larger studies is needed to confirm these results and compare this treatment to other options.
Conflict of interest statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures


References
-
- Watanabe J, Muro K, Shitara K, et al. Panitumumab vs Bevacizumab added to standard first-line chemotherapy and overall survival among patients with RAS Wild-type, left-sided metastatic colorectal cancer: a randomized clinical trial. JAMA. 2023;329(15):1271–1282. doi: 10.1001/jama.2023.4428 - DOI - PMC - PubMed
-
- Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (alliance). JCO. 2016;34(15_suppl):3504–3504. doi: 10.1200/JCO.2016.34.15_suppl.3504 - DOI
-
- Stintzing S, Modest DP, Rossius L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;17(10):1426–1434. doi: 10.1016/S1470-2045(16)30269-8 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
