Parkinson's disease mutant Miro1 causes mitochondrial dysfunction and dopaminergic neuron loss
- PMID: 39913247
- PMCID: PMC12493065
- DOI: 10.1093/brain/awaf051
Parkinson's disease mutant Miro1 causes mitochondrial dysfunction and dopaminergic neuron loss
Abstract
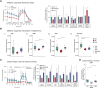
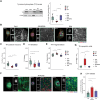
The complex and heterogeneous nature of Parkinson's disease (PD) is still not fully understood. However, increasing evidence supports mitochondrial impairment as a major driver of neurodegeneration. Miro1, a mitochondrial GTPase encoded by the RHOT1 gene, is involved in mitochondrial transport, mitophagy and mitochondrial calcium buffering, and is therefore essential for maintaining mitochondrial homeostasis. Recently, Miro1 has been linked genetically and pathophysiologically to PD, further supported by the identification of heterozygous variants of Miro1 in patients. Herein, we used patient-derived cellular models alongside knock-in mice to investigate Miro1-dependent pathophysiological processes and molecular mechanisms underlying neurodegeneration in PD. Experimental work performed in induced pluripotent stem cell (iPSC)-derived models, including midbrain organoids and dopaminergic neuronal cell cultures from a PD patient carrying the p.R272Q Miro1 mutation as well as healthy and isogenic controls, indicated that the p.R272Q Miro1 mutation leads to increased oxidative stress, disrupted mitochondrial bioenergetics and altered cellular metabolism. These changes were accompanied by increased α-synuclein levels and a significant reduction of dopaminergic neurons. Moreover, the p.R272Q Miro1 mutation-located in the calcium-binding domain of the GTPase-disrupted calcium homeostasis, resulting in calcium-dependent activation of calpain proteases and the subsequent cleavage of α-synuclein. Knock-in mice expressing p.R285Q Miro1 (the murine orthologue of the human p.R272Q mutation) displayed accumulation of phosphorylated α-synuclein in the striatum and a significant loss of dopaminergic neurons in the substantia nigra pars compacta, accompanied by behavioural alterations. These findings demonstrate that mutant Miro1 is sufficient to comprehensively model PD-relevant phenotypes in vitro and in vivo, reinforcing its pivotal role in PD pathogenesis.
Keywords: calcium homeostasis; knock-in mice; neurodegeneration; p.R272Q Miro1; patient-specific iPSC-derived models; α-synuclein.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
J.C.S. is co-inventor on a patent (WO2017060884A1) describing the midbrain organoid technology used and co-founder of OrganoTherapeutics. The other authors report no competing interests.
Figures




References
-
- Antony PMA, Diederich NJ, Krüger R, Balling R. The hallmarks of Parkinson’s disease. FEBS J. 2013;280:5981–5993. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

