Drug-induced autoimmune-like hepatitis: A disproportionality analysis based on the FAERS database
- PMID: 39913410
- PMCID: PMC11801597
- DOI: 10.1371/journal.pone.0317680
Drug-induced autoimmune-like hepatitis: A disproportionality analysis based on the FAERS database
Abstract
Background: Drug-induced autoimmune-like hepatitis (DI-ALH) is a potentially life-threatening condition that can lead to acute liver failure and necessitate liver transplantation. While the association between certain drugs and DI-ALH has been documented, a comprehensive analysis of drug-related signals in a large, real-world pharmacovigilance database is lacking. This study aimed to systematically identify drugs linked to DI-ALH by analyzing adverse event reports from the U.S. Food and Drug Administration's (FDA) Adverse Event Reporting System (FAERS) database.
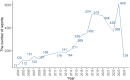
Methods: We searched the FAERS database for the term "autoimmune hepatitis" and extracted DI-ALH reports from the first quarter of 2004 to the first quarter of 2024. Positive signal drugs were identified using Proportional Reporting Ratio (PRR), Reporting Odds Ratio (ROR), Bayesian Confidence Propagation Neural Network (BCPNN), and Empirical Bayesian Geometric Mean (EBGM). To confirm a significant drug-adverse event association, each method had to meet predefined thresholds: for PRR and ROR, values were considered significant if the lower 95% confidence interval (CI) was greater than 1 and at least three reports were identified; for BCPNN, an Information Component (IC025) greater than 0 indicated a signal; for EBGM, a value greater than 2 for the lower 95% confidence interval (EBGM05) was used to denote a positive signal.
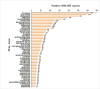
Results: A total of 5,723 DI-ALH reports were extracted from the FAERS database. Disproportionality analysis identified 50 drugs with strong associations to DI-ALH, with biologics, statins, antibiotics, and antiviral drugs representing the most common categories. Among these, nitrofurantoin (ROR 94.79, CI 78.53-114.41), minocycline (ROR 77.82, CI 65.09-93.05), and nivolumab (ROR 47.12, CI 15.06-147.39) exhibited the strongest signals. Additionally, several previously unreported drugs, including mesalazine, aldesleukin, onasemnogene abeparvovec-xioi, and nefazodone, were identified as having strong associations with DI-ALH. These findings were consistent across all four signal detection methods, further validating the robustness of the associations.
Conclusion: This study provides a comprehensive assessment of drugs associated with DI-ALH through a rigorous analysis of the FAERS database using multiple signal detection methods. By identifying both well-known and previously underreported drugs, this study contributes to a more complete understanding of drug-induced liver injury. The findings have important implications for pharmacovigilance strategies and clinical risk assessment. However, limitations inherent in the FAERS database, such as underreporting and the potential for reporting bias, should be considered. Further clinical validation is warranted to confirm these associations.
Copyright: © 2025 Ye et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Post-marketing safety profile of ganirelix in women: a 20-year pharmacovigilance analysis of global adverse drug event databases (2004-2024).BMC Pharmacol Toxicol. 2025 Apr 22;26(1):91. doi: 10.1186/s40360-025-00920-4. BMC Pharmacol Toxicol. 2025. PMID: 40264185 Free PMC article.
-
Post-market safety profile of cefiderocol: a real-world pharmacovigilance exploratory analysis based on U.S. FDA adverse event reporting system (FAERS).BMC Pharmacol Toxicol. 2025 Mar 11;26(1):58. doi: 10.1186/s40360-025-00894-3. BMC Pharmacol Toxicol. 2025. PMID: 40069825 Free PMC article.
-
Signal mining and analysis for central nervous system adverse events due to taking oxycodone based on FAERS database.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023 Mar 28;48(3):422-434. doi: 10.11817/j.issn.1672-7347.2023.220304. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37164926 Free PMC article. Chinese, English.
-
Romosozumab adverse event profile: a pharmacovigilance analysis based on the FDA Adverse Event Reporting System (FAERS) from 2019 to 2023.Aging Clin Exp Res. 2025 Jan 14;37(1):23. doi: 10.1007/s40520-024-02921-5. Aging Clin Exp Res. 2025. PMID: 39808360 Free PMC article.
-
Adverse drug reactions related to methotrexate: a real-world pharmacovigilance study using the FAERS database from 2004 to 2024.Front Immunol. 2025 Jun 4;16:1586361. doi: 10.3389/fimmu.2025.1586361. eCollection 2025. Front Immunol. 2025. PMID: 40534848 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical