Activating PKC-ε induces HIV expression with improved tolerability
- PMID: 39913544
- PMCID: PMC11801715
- DOI: 10.1371/journal.ppat.1012874
Activating PKC-ε induces HIV expression with improved tolerability
Abstract
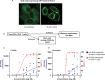
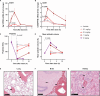
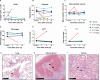
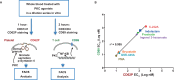
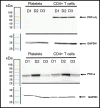
Despite suppressive antiretroviral therapy (ART), HIV-1 persists in latent reservoirs that seed new HIV infections if ART is interrupted, necessitating lifelong therapy for people with HIV. Activation of latent HIV during ART could improve recognition and elimination of infected cells by the immune system. Protein kinase C (PKC) isozymes increase HIV transcription and hence are potential latency reversal agents. However, the clinical utility of PKCs for this application is limited due to toxicity, which is poorly understood. Our studies showed that PKC activation with multiple classes of agonists leads to widespread platelet activation, consistent with disseminated intravascular coagulation, at concentrations that were similar to those required for T-cell activation. Differential expression analysis indicated that PKC-ε and PKC-η isoforms are expressed at high levels in human CD4+ T cells but not in platelets. Using structure-based drug design, we developed a novel PKC agonist, C-233, with increased selectivity for PKC-ε. C-233 increased both supernatant HIV RNA and p24 expression ex vivo after treatment of CD4+ T cells from ART-suppressed people with HIV. C-233 was 5-fold more potent for T-cell activation relative to platelet activation. Our studies support the use of structure-based drug design to create selective novel PKC agonists for the safe activation of HIV reservoirs and improved tolerability.
Copyright: © 2025 Irrinki et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: At the time of this work, all authors, with the exception of SD and JL, were employees and shareholders of Gilead Sciences, Inc. RM, JK, HY, BS, HT, EH, and JPM are inventors on PKC agonist–related patent applications. Authors SD and JL declare that no competing interests exist.
Figures





References
-
- Debrabander Q, Hensley KS, Psomas CK, Bramer W, Mahmoudi T, van Welzen BJ, et al.. The efficacy and tolerability of latency-reversing agents in reactivating the HIV-1 reservoir in clinical studies: a systematic review. J Virus Erad. 2023;9(3):100342. doi: 10.1016/j.jve.2023.100342 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

