Disrupting AGR2/IGF1 paracrine and reciprocal signaling for pancreatic cancer therapy
- PMID: 39914384
- PMCID: PMC11866503
- DOI: 10.1016/j.xcrm.2024.101927
Disrupting AGR2/IGF1 paracrine and reciprocal signaling for pancreatic cancer therapy
Abstract
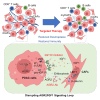
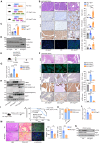
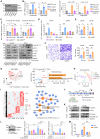
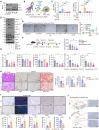
Pancreatic ductal adenocarcinoma (PDAC) is highly aggressive and characterized by pronounced desmoplasia. PDAC cells communicate with cancer-associated fibroblasts (CAFs) in a paracrine/reciprocal manner, substantially promoting tumor growth and desmoplastic responses. This study highlights the critical role of anterior gradient 2 (AGR2), an endoplasmic reticulum protein disulfide isomerase, secreted by PDAC cells to activate CAFs via the Wnt signaling pathway. Activated CAFs, in turn, secrete insulin-like growth factor 1 (IGF1), which enhances AGR2 expression and secretion in PDAC cells through the IGF1 receptor (IGF1R)/c-JUN axis. Within PDAC cells, AGR2 acts as a thioredoxin, aiding the folding and cell surface presentation of IGF1R, essential for PDAC's response to CAF-derived IGF1. This reciprocal AGR2/IGF1 signaling loop intensifies desmoplasia, immunosuppression, and tumorigenesis, creating a harmful feedback loop. Targeting both pathways disrupts this interaction, reduces desmoplasia, and restores anti-tumor immunity in preclinical models, offering a promising therapeutic strategy against PDAC.
Keywords: AGR2; IGF1; IGF1R; desmoplastic reaction; immunosuppression; molecular targeting; pancreatic cancer.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Tape C.J., Ling S., Dimitriadi M., McMahon K.M., Worboys J.D., Leong H.S., Norrie I.C., Miller C.J., Poulogiannis G., Lauffenburger D.A., Jørgensen C. Oncogenic KRAS Regulates Tumor Cell Signaling via Stromal Reciprocation. Cell. 2016;165:910–920. doi: 10.1016/j.cell.2016.03.029. - DOI - PMC - PubMed
-
- Olive K.P., Jacobetz M.A., Davidson C.J., Gopinathan A., McIntyre D., Honess D., Madhu B., Goldgraben M.A., Caldwell M.E., Allard D., et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. - DOI - PMC - PubMed
-
- Ireland L., Santos A., Ahmed M.S., Rainer C., Nielsen S.R., Quaranta V., Weyer-Czernilofsky U., Engle D.D., Perez-Mancera P.A., Coupland S.E., et al. Chemoresistance in Pancreatic Cancer Is Driven by Stroma-Derived Insulin-Like Growth Factors. Cancer Res. 2016;76:6851–6863. doi: 10.1158/0008-5472.CAN-16-1201. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

