Granzyme K activates the entire complement cascade
- PMID: 39914456
- PMCID: PMC12180478
- DOI: 10.1038/s41586-025-08713-9
Granzyme K activates the entire complement cascade
Abstract
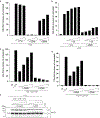
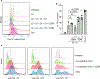
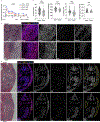
Granzymes are a family of serine proteases that are mainly expressed by CD8+ T cells, natural killer cells and innate-like lymphocytes1. Although their primary function is thought to be the induction of cell death in virally infected cells and tumours, accumulating evidence indicates that some granzymes can elicit inflammation by acting on extracellular substrates1. We previously found that most tissue CD8+ T cells in rheumatoid arthritis synovium, and in inflamed organs for some other diseases, express granzyme K (GZMK)2, a tryptase-like protease with poorly defined function. Here, we show that GZMK can activate the complement cascade by cleaving the C2 and C4 proteins. The nascent C4b and C2b fragments form a C3 convertase that cleaves C3, enabling the assembly of a C5 convertase that cleaves C5. The resulting convertases generate all the effector molecules of the complement cascade: the anaphylatoxins C3a and C5a, the opsonins C4b and C3b, and the membrane attack complex. In rheumatoid arthritis synovium, GZMK is enriched in regions with abundant complement activation, and fibroblasts are the main producers of complement proteins that serve as substrates for GZMK-mediated complement activation. Furthermore, Gzmk-deficient mice are significantly protected from inflammatory disease, exhibiting reduced arthritis and dermatitis, with concomitant decreases in complement activation. Our findings describe the discovery of a previously unidentified mechanism of complement activation that is driven entirely by lymphocyte-derived GZMK. Given the widespread abundance of GZMK-expressing T cells in tissues in chronic inflammatory diseases, GZMK-mediated complement activation is likely to be an important contributor to tissue inflammation in multiple disease contexts.
© 2025. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: M.B.B. is a consultant to GSK, Third Rock Ventures, 4FO Ventures and Moderna and a consultant to and founder of Mestag Therapeutics. S.R. is a founder of Mestag Therapeutics, a scientific adviser for Janssen and Pfizer, and a consultant to Gilead and Rheos Medicine. D.F.D. is a consultant to Celldex Therapeutics. The remaining authors declare no competing interests.
Figures





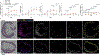





Update of
-
Granzyme K drives a newly-intentified pathway of complement activation.bioRxiv [Preprint]. 2024 May 26:2024.05.22.595315. doi: 10.1101/2024.05.22.595315. bioRxiv. 2024. Update in: Nature. 2025 May;641(8061):211-221. doi: 10.1038/s41586-025-08713-9. PMID: 38826230 Free PMC article. Updated. Preprint.
References
-
- Anthony DA, Andrews DM, Watt SV, Trapani JA & Smyth MJ Functional dissection of the granzyme family: cell death and inflammation. Immunol. Rev. 235, 73–92 (2010). - PubMed
MeSH terms
Substances
Grants and funding
- P01 AI148102/AI/NIAID NIH HHS/United States
- UH2 AR067685/AR/NIAMS NIH HHS/United States
- R01 AR081792/AR/NIAMS NIH HHS/United States
- K08 AR081412/AR/NIAMS NIH HHS/United States
- UH2 AR067681/AR/NIAMS NIH HHS/United States
- UH2 AR067688/AR/NIAMS NIH HHS/United States
- UH2 AR067689/AR/NIAMS NIH HHS/United States
- UH2 AR067677/AR/NIAMS NIH HHS/United States
- UH2 AR067694/AR/NIAMS NIH HHS/United States
- UH2 AR067679/AR/NIAMS NIH HHS/United States
- T32 AR007098/AR/NIAMS NIH HHS/United States
- UM2 AR067678/AR/NIAMS NIH HHS/United States
- R01 AR073290/AR/NIAMS NIH HHS/United States
- UC2 AR081023/AR/NIAMS NIH HHS/United States
- R01 AR063759/AR/NIAMS NIH HHS/United States
- UH2 AR067690/AR/NIAMS NIH HHS/United States
- UH2 AR067676/AR/NIAMS NIH HHS/United States
- UH2 AR067691/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

