Multicentre, 26-week, open-label phase 2 trial of the JAK inhibitor filgotinib in Behçet's disease, idiopathic inflammatory myopathies and IgG4-related disease: DRIMID study protocol
- PMID: 39915014
- PMCID: PMC11815459
- DOI: 10.1136/bmjopen-2024-089827
Multicentre, 26-week, open-label phase 2 trial of the JAK inhibitor filgotinib in Behçet's disease, idiopathic inflammatory myopathies and IgG4-related disease: DRIMID study protocol
Abstract
Introduction: Research into novel therapies for rare, immune-mediated inflammatory diseases (IMIDs) faces significant challenges, including small patient populations, complex clinical trial design and difficulties in patient recruitment. Patients with Behçet's disease (BD), idiopathic inflammatory myopathies (IIM) and IgG4-related disease (IgG4-RD) typically undergo treatment involving prolonged administration of high-dose glucocorticoids and immunosuppressants. Both are associated with an increased risk of infection. Additionally, glucocorticoids carry long-term toxicity risks. Thus, there is an urgent need to develop more targeted and effective anti-inflammatory treatments. Given the activation of the type 1 interferon pathway in BD, IIM and IgG4-RD, inhibition of the Janus kinase (JAK) STAT pathway emerges as a promising therapeutic strategy. The Drug Rediscovery in IMIDs (DRIMID) consortium aims to conduct a prospective pilot basket trial to investigate the effects of filgotinib, a JAK1 preferential inhibitor approved for ulcerative colitis and rheumatoid arthritis, on disease activity, quality of life and safety in patients with refractory BD, IIM and IgG4-RD.
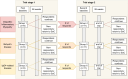
Methods and analysis: In this investigator-initiated, multicentre, open-label phase 2 study, up to 60 patients with rare IMIDs will be enrolled for a 26-week treatment period with filgotinib 200 mg once daily. The trial consists of two stages, each involving a consecutively treated cohort of up to 20 patients per disease. An interim analysis is conducted between these stages, where the trial will proceed only in diseases showing potential effectiveness. Baseline, 3-month and 6-month assessments will include data on quality of life, disease activity, corticosteroid toxicity and biomarkers. The coprimary endpoints are disease activity and quality of life across and within each disease.
Ethics and dissemination: The study received approval from the Medical Research Ethics Committee in Utrecht, Netherlands. A Data Safety Monitoring Board has been established to monitor the trial's safety and progress.
Trial registration number: NCT06285539.
Keywords: Clinical Trial; IMMUNOLOGY; Patient Reported Outcome Measures; Patients; Quality of Life; Rheumatology.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: NHR and EP are employees of Alfasigma S.p.A. and were formerly employed by Galápagos NV. JMvL has received consultancy honoraria from Galápagos NV. RJG has received a speaker fee, and the pharmacy of his institution has received an institutional grant from Galápagos NV. BCG-H, JAMvL, JR, SWT, PMJW, CMC and RMT declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous