International Severe Asthma Registry (ISAR): 2017-2024 Status and Progress Update
- PMID: 39915034
- PMCID: PMC12010726
- DOI: 10.4046/trd.2024.0198
International Severe Asthma Registry (ISAR): 2017-2024 Status and Progress Update
Abstract
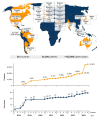
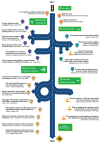
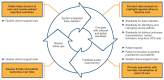
The International Severe Asthma Registry (ISAR) was established in 2017 to advance the understanding of severe asthma and its management, thereby improving patient care worldwide. As the first global registry for adults with severe asthma, ISAR enabled individual registries to standardize and pool their data, creating a comprehensive, harmonized dataset with sufficient statistical power to address key research questions and knowledge gaps. Today, ISAR is the largest repository of real-world data on severe asthma, curating data on nearly 35,000 patients from 28 countries worldwide, and has become a leading contributor to severe asthma research. Research using ISAR data has provided valuable insights on the characteristics of severe asthma, its burdens and risk factors, real-world treatment effectiveness, and barriers to specialist care, which are collectively informing improved asthma management. Besides changing clinical thinking via research, ISAR aims to advance real-world practice through initiatives that improve registry data quality and severe asthma care. In 2024, ISAR refined essential research variables to enhance data quality and launched a web-based data acquisition and reporting system (QISAR), which integrates data collection with clinical consultations and enables longitudinal data tracking at patient, center, and population levels. Quality improvement priorities include collecting standardized data during consultations and tracking and optimizing patient journeys via QISAR and integrating primary/secondary care pathways to expedite specialist severe asthma management and facilitate clinical trial recruitment. ISAR envisions a future in which timely specialist referral and initiation of biologic therapy can obviate long-term systemic corticosteroid use and enable more patients to achieve remission.
Keywords: Core Variables; Delphi Consensus; International Severe Asthma Registry (ISAR); Optimum Patient Care Global; Quality Improvement; Real-World Data.
Conflict of interest statement
Désirée Larenas-Linnemann reports personal fees from ALK-Abelló, AstraZeneca national and global, Bayer, Chiesi, Grunenthal, Grin, GlaxoSmithKline national and global, Viatris, Novartis, Pfizer, Sanofi, Siegfried, and Carnot, and grants for guideline development from Abbvie, Bayer, Chiesi, Lilly, Sanofi, AstraZeneca, Pfizer, Novartis, Circassia, UCB, and GlaxoSmithKline, outside the submitted work.
Chin Kook Rhee received consulting/lecture fees from Merck Sharp & Dohme, AstraZeneca, GlaxoSmith-Kline, Novartis, Takeda, Mundipharma, Boehringer Ingelheim, Teva, Sanofi, Organon, Roche, and Bayer.
Alan Altraja has received lecture fees from AstraZeneca, Berlin-Chemie Menarini, Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, Merck Sharp & Dohme, Norameda, Novartis, Orion, Sanofi, and Zentiva; sponsorships from AstraZeneca, Berlin-Chemie Menarini, Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, Merck Sharp & Dohme, Norameda, Novartis, and Sanofi; and has participated in advisory boards for AstraZeneca, Boehringer Ingelheim, CSL Behring, GlaxoSmith-Kline, Merck Sharp&Dohme, Novartis, Sanofi, Shire Pharmaceuticals, and Teva.
John Busby has received research grants from Astra-Zeneca and personnel fees from NuvoAir, outside the submitted work.
Trung N. Tran is an employee of AstraZeneca and may own stocks or stock options in AstraZeneca. Astra-Zeneca is a co-funder of the International Severe Asthma Registry (ISAR).
Eileen Wang has received honoraria from AstraZeneca, GlaxoSmithKline, Amgen, and Genentech. She has been an investigator on studies sponsored by Astra-Zeneca, GlaxoSmithKline, Genentech, and Sanofi, for which her institution has received funding.
Todor A. Popov declares research support from Novartis and Chiesi Pharma, outside the submitted work.
Patrick D. Mitchell has received speaker fees from GlaxoSmithKline, AstraZeneca, Teva and Novartis, and has received grants from AstraZeneca, and Teva. He has received advisor board fees from AstraZeneca.
Paul E. Pfeffer has attended advisory boards for AstraZeneca, GlaxoSmithKline, and Sanofi; has given lectures/webinars at meetings supported by AstraZeneca, Chiesi, and GlaxoSmithKline; has taken part in clinical trials sponsored by AstraZeneca, GlaxoSmithKline, Novartis, Regeneron, and Sanofi, for which his institution received remuneration; and has current research grants funded by GlaxoSmithKline and a quality improvement grant funded by AstraZeneca.
Roy Alton Pleasants is a consultant for AstraZeneca and Grifols and receives research support through institutions from AstraZeneca and GlaxoSmithKline.
Rohit Katial declares honoraria from Amgen, Astra-Zeneca, Sanofi/Regeneron, GlaxoSmithKline, and Grifols.
Mariko Siyue Koh reports grant support from Astra-Zeneca, and honoraria for lectures and advisory board meetings paid to her hospital (Singapore General Hospital) from GlaxoSmithKline, AstraZeneca, Novartis, Sanofi, and Boehringer Ingelheim, outside the submitted work.
Arnaud Bourdin has received industry-sponsored grants from AstraZeneca/MedImmune, Boehringer Ingelheim, Cephalon/Teva, GlaxoSmithKline, Novartis, Sanofi-Regeneron, and has consultancies with AstraZeneca/MedImmune, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Regeneron-Sanofi, Med-in-Cell, Actelion, Merck, Roche, and Chiesi.
Florence Schleich reports consultancy work for GlaxoSmithKline, AstraZeneca, Sanofi- Advisory board, received speaker fees from GlaxoSmithKline, AstraZeneca, Chiesi, Teva, ALK-Abelló, and research grants from GlaxoSmithKline, AstraZeneca, and Chiesi.
Jorge Máspero reports speaker fees, grants, or advisory boards for AstraZeneca, Sanofi, GlaxoSmithKline, Novartis, Inmunotek, Menarini, and Noucor.
Mark Hew declares grants and other advisory board fees (made to his institutional employer) from Astra-Zeneca, GlaxoSmithKline, Novartis, Sanofi, Teva, and Seqirus, for unrelated projects.
Matthew J. Peters declares personal fees and non-financial support from AstraZeneca, GlaxoSmithKline, and Sanofi.
David J. Jackson has received speaker fees and consultancy fees from AstraZeneca, GlaxoSmithKline, Sanofi Regeneron, and Boehringer Ingelheim, and research funding from AstraZeneca.
George C. Christoff declares relevant research support from AstraZeneca and Sanofi.
Luis Perez-de-Llano reports grants, personal fees, and non-financial support from AstraZeneca; personal fees and non-financial support from GlaxoSmithKline; grants, personal fees and non-financial support from Teva; personal fees and non-financial support from Chiesi; grants, personal fees and non-financial support from Sanofi; personal fees from Merck Sharp&Dohme; personal fees from Techdow Pharma; grants, personal fees and non-financial support from Faes Farma; personal fees from Leo-Pharma; grants and personal fees from Gebro; personal fees from Gilead, outside the submitted work.
Ivan Cherrez-Ojeda declares no conflict of interest.
João A. Fonseca reports grants from research agreements with AstraZeneca, Mundipharma, Sanofi Regeneron, and Novartis. Personal fees for lectures and attending advisory boards: AstraZeneca, GlaxoSmithKline, Mundipharma, Novartis, Sanofi Regeneron, and Teva.
Richard W. Costello has received honoraria for lectures from Aerogen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Teva. He is a member of advisory boards for GlaxoSmithKline and Novartis, has received grant support from GlaxoSmith-Kline and Aerogen, and has patents in the use of acoustics in the diagnosis of lung disease, assessment of adherence and prediction of exacerbations.
Carlos A. Torres-Duque has received fees as advisory board participant and/or speaker from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Sanofi-Aventis; has taken part in clinical trials from AstraZeneca, Novartis and Sanofi-Aventis; has received unrestricted grants for investigator-initiated studies at Fundacion Neumologica Colombiana from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Grifols, and Novartis.
Piotr Kuna reports personal fees from Adamed, Astra-Zeneca, Berlin Chemie Menarini, FAES, Glenmark, NoPiotr Kuna reports personal fees from Adamed, Astra-Zeneca, Berlin Chemie Menarini, FAES, Glenmark, Novartis, Polpharma, Boehringer Ingelheim, Teva, Zentiva, outside the submitted work.
Andrew N. Menzies-Gow is an employee of AstraZeneca and may own stock or stock options in AstraZeneca. AstraZeneca is a co-funder of ISAR.
Neda Stjepanovic is an employee of AstraZeneca and may own stock options in AstraZeneca. AstraZeneca is a co-funder of ISAR.
Peter G. Gibson has received speaker fees and grants to his institution from AstraZeneca, GlaxoSmithKline, and Novartis.
Paulo Márcio Pitrez received fees as a speaker or for consultations from GlaxoSmithKline, AstraZeneca, Sanofi, and Aché.
Celine Bergeron reports advisory board participation of Sanofi-Regeneron, AstraZeneca, Takeda, ValeoPharma, consultant for Areteia, honorarium for presentations for AstraZeneca/Amgen, GlaxoSmithKline, Grifols, Sanofi-Regeneron, ValeoPharma and grants paid to The University of British Columbia from BioHaven, Sanofi-Regeneron, AstraZeneca, and GlaxoSmithKline.
Celeste M. Porsbjerg has attended advisory boards for AstraZeneca, Novartis, TEVA, and Sanofi-Genzyme; has given lectures at meetings supported by AstraZeneca, Novartis, TEVA, Sanofi-Genzyme, and GlaxoSmith-Kline; has taken part in clinical trials sponsored by AstraZeneca, Novartis, Merck Sharp&Dohme, Sanofi-Genzyme, GlaxoSmithKline, and Novartis; and has received educational and research grants from Astra-Zeneca, Novartis, TEVA, GlaxoSmithKline, ALK-Abelló, and Sanofi-Genzyme.
Camille Taillé has received lecture or advisory board fees and grants to her institution from AstraZeneca, Sanofi, GlaxoSmithKline, Chiesi, Stallergenes, and Novartis, for unrelated projects.
Christian Taube declares no relevant conflict of interest.
Nikolaos G. Papadopoulos has been a speaker and/or advisory board member for Abbott, Abbvie, ALK-Abelló, Asit Biotech, AstraZeneca, Biomay, Boehringer Ingelheim, GlaxoSmithKline, HAL, Faes Farma, Medscape, Menarini, Merck Sharp&Dohme, Novartis, Nutricia, OM Pharma, Regeneron, Sanofi, Takeda, and Viatris.
Andriana I. Papaioannou has received fees and honoraria from Menarini, GlaxoSmithKline, Novartis, Elpen, Boehringer Ingelheim, AstraZeneca, and Chiesi.
Sundeep Salvi declares research support and speaker fees from Cipla, Glenmark, and GlaxoSmithKline.
Giorgio Walter Canonica has received research grants, as well as lecture or advisory board fees from A. Menarini, ALK-Abelló, Allergy Therapeutics, Anallergo, AstraZeneca, MedImmune, Boehringer Ingelheim, Chiesi Farmaceutici, Circassia, Danone, Faes, Genentech, Guidotti Malesci, GlaxoSmithKline, Hal Allergy, Merck, Merck Sharp&Dohme, Mundipharma, Novartis, Orion, Sanofi Aventis, Sanofi, Genzyme/Regeneron, Stallergenes, UCB Pharma, Uriach Pharma, Teva, Thermo Fisher, and Valeas.
Enrico Heffler declares personal fees for advisory boards participation and/or speaker activities from: Sanofi, Regeneron, GlaxoSmithKline, Novartis, Astra-Zeneca, Stallergenes-Greer, Circassia, Bosch, Celltrion-Healthcare, Chiesi, and Almirall.
Takashi Iwanaga received speaker bureau fees from Kyorin, GlaxoSmithKline, Novartis, Boehringer Ingelheim, AstraZeneca, and Sanofi.
Mona S. Al-Ahmad has received advisory board and speaker fees from AstraZeneca, Sanofi, Novartis, and GlaxoSmithKline, and received a grant from Kuwait Foundation for the Advancement of Sciences (KFAS).
Sverre Lehmann has been an investigator on clinical trials sponsored by GlaxoSmithKline and AstraZeneca, for which his institution has received funding.
Riyad Al-Lehebi has given lectures at meetings supported by AstraZeneca, Boehringer Ingelheim, Novartis, GlaxoSmithKline, and Sanofi, and participated in advisory board fees from GlaxoSmithKline, AstraZeneca, Novartis, and Abbott.
Borja G. Cosio declares grants from Chiesi, Menarini, and GlaxoSmithKline; personal fees for advisory board activities from Chiesi, GlaxoSmithKline, Novartis, Sanofi, Teva, and AstraZeneca; and payment for lectures/speaking engagements from Sanofi, Chiesi, Novartis, GlaxoSmithKline, Menarini, and AstraZeneca, outside the submitted work.
Diahn-Warng Perng received sponsorship to attend or speak at international meetings, honoraria for lecturing or attending advisory boards, and research grants from the following companies: AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Daiichi Sankyo, Shionogi, and Orient Pharma.
Bassam Mahboub reports no conflict of interest.
Liam G. Heaney has received grant funding, participated in advisory boards and given lectures at meetings supported by Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Hoffmann la Roche, GlaxoSmithKline, Novartis, Theravance, Evelo Biosciences, Sanofi, and Teva; he has received grants from MedImmune, Novartis UK, Roche/Genentech Inc., GlaxoSmithKline, Amgen, Genentech/Hoffman la Roche, AstraZeneca, MedImmune, Aerocrine, and Vitalograph; he has received sponsorship for attending international scientific meetings from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Napp Pharmaceuticals; he has also taken part in asthma clinical trials sponsored by AstraZeneca, Boehringer Ingelheim, Hoffmann la Roche, and GlaxoSmithKline for which his institution received remuneration; he is the Academic Lead for the Medical Research Council Stratified Medicine UK Consortium in Severe Asthma which involves industrial partnerships with a number of pharmaceutical companies including Amgen, Astra-Zeneca, Boehringer Ingelheim, GlaxoSmithKline, Hoffmann la Roche, and Janssen.
Pujan H. Patel has received advisory board and speaker fees from AstraZeneca, GlaxoSmithKline, Novartis, and Sanofi/Regeneron.
Njira Lugogo received consulting fees from Amgen, AstraZeneca, Avillion, Genentech, GlaxoSmithKline, Niox, Novartis, Regeneron, Sanofi, and Teva; honoraria for non-speakers bureau presentations from GlaxoSmithKline, TEVA and AstraZeneca; and travel support from AstraZeneca, SANOFI, TEVA, Regeneron and GlaxoSmithKline; her institution received research support from Amgen, AstraZeneca, Avillion, Bellus, Evidera, Gossamer Bio, Genentech, GlaxoSmithKline, Janssen, Niox, Regeneron, Sanofi, Novartis, and Teva. She is an honorary faculty member of Observational and Pragmatic Research Institute (OPRI) but does not receive compensation for this role.
Michael E. Wechsler reports grants and/or personal fees from Novartis, Sanofi, Regeneron, Genentech, Sentien, Restorbio, Equillium, Genzyme, Cohero Health, Teva, Boehringer Ingelheim, AstraZeneca, Amgen, GlaxoSmithKline, Cytoreason, Cerecor, Sound Biologics, Incyte, and Kinaset.
Lakmini Bulathsinhala is an employee of the OPRI. OPRI conducted this study in collaboration with Optimum Patient Care (OPC), a co-funder of the ISAR.
Victoria Carter is an employee of OPC. OPC is a cofunder of the ISAR.
Kirsty Fletton is an employee of Optimum Patient Care Global (OPCG), a co-funder of the ISAR.
David L. Neil is an employee of the OPRI. OPRI conducted this study in collaboration with OPC, a cofunder of the ISAR.
Ghislaine Scelo is a consultant for OPRI. OPRI conducted this study in collaboration with OPC, a cofunder of the ISAR.
David B. Price has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; consultancy agreements with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; grants and unrestricted funding for investigator-initiated studies (conducted through OPRI Pte Ltd.) from AstraZeneca, Chiesi, Viatris, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Inside Practice, GlaxoSmithKline, Medscape, Viatris, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme, Teva Pharmaceuticals; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Novartis, Medscape, Teva Pharmaceuticals.; owns 74% of the social enterprise OPC Ltd. (Australia and UK) and 92.61% of OPRI Pte Ltd. (Singapore); is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation Programme, and Health Technology Assessment; and he was an expert witness for GlaxoSmithKline.
Figures

References
-
- ISAR Study Group International Severe Asthma Registry: mission statement. Chest. 2020;157:805–14. - PubMed
-
- Bulathsinhala L, Eleangovan N, Heaney LG, Menzies-Gow A, Gibson PG, Peters M, et al. Development of the International Severe Asthma Registry (ISAR): a modified Delphi study. J Allergy Clin Immunol Pract. 2019;7:578–88. - PubMed
-
- Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC, Menzies-Gow AN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry. Chest. 2020;157:790–804. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

