YAP/TAZ are crucial regulators of macrophage-mediated pulmonary inflammation and fibrosis after bleomycin-induced injury
- PMID: 39915054
- PMCID: PMC12138047
- DOI: 10.1183/13993003.01544-2023
YAP/TAZ are crucial regulators of macrophage-mediated pulmonary inflammation and fibrosis after bleomycin-induced injury
Abstract
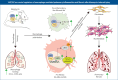
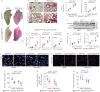
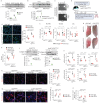
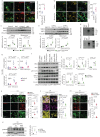
Pulmonary fibrosis is the most prevalent and severe form of end-stage interstitial lung disease. Macrophages are crucial players in inflammation-induced pulmonary fibrosis, but the mechanisms driving macrophage polarisation and their specific roles in pulmonary fibrosis pathogenesis remain poorly understood. Here, we demonstrate that both YAP and TAZ are activated in lung macrophages from patients with pulmonary fibrosis as well as in mice with bleomycin-induced pulmonary fibrosis. Myeloid-specific Yap/Taz deletion resulted in reduced recruitment of monocyte-derived alveolar macrophages (Mo-AMs), impaired inflammatory responses, decreased pulmonary fibrosis and enhanced alveolar epithelial cell regeneration following bleomycin treatment. Conversely, the expression of a constitutively active YAP mutant (YAP5SA) exacerbated bleomycin-induced pulmonary fibrosis by increasing Mo-AM recruitment, elevating expression of pro-inflammatory and pro-fibrotic markers, and impairing alveolar epithelial cell regeneration. We demonstrate that YAP/TAZ-CCL2 (C-C motif chemokine ligand 2) signalling plays a crucial role in bleomycin-induced pulmonary fibrosis, as blocking CCL2 with a neutralising antibody effectively abrogated the YAP5SA-induced recruitment of Mo-AMs, inflammatory and fibrotic responses. Additionally, we reveal that the YAP/TAZ-MBD2-TGFβ1-pSMAD2 signalling axis is crucial not only for pro-fibrotic macrophage polarisation, but also for their cross-talk with lung fibroblasts, driving the fibroblast-to-myofibroblast transition. Collectively, these findings suggest that targeting aberrant YAP/TAZ activity to modulate inflammatory and fibrotic response could be a promising strategy for the prevention and treatment of pulmonary fibrosis.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: The authors have no potential conflicts of interest to disclose.
Figures





Comment in
-
Yapping about macrophages in fibrotic lung disease.Eur Respir J. 2025 Jun 5;65(6):2500075. doi: 10.1183/13993003.00075-2025. Print 2025 Jun. Eur Respir J. 2025. PMID: 40473297 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
