Safety, Dosimetry, and Feasibility of [68Ga]Ga-PSMA-R2 as an Imaging Agent in Patients with Biochemical Recurrence or Metastatic Prostate Cancer
- PMID: 39915126
- PMCID: PMC11876733
- DOI: 10.2967/jnumed.124.268318
Safety, Dosimetry, and Feasibility of [68Ga]Ga-PSMA-R2 as an Imaging Agent in Patients with Biochemical Recurrence or Metastatic Prostate Cancer
Abstract
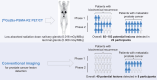
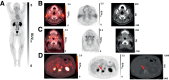
Prostate-specific membrane antigen (PSMA) is highly expressed in most prostate cancers (PCs). PET and CT imaging studies using 68Ga-labeled PSMA ligands demonstrated the specific localization of 68Ga in PC lesions and distant metastatic lesions. [68Ga]Ga-PSMA-R2 (68Ga-PSMA-R2) is a PSMA-targeted PET/CT radiotracer with potential diagnostic applications. Methods: PROfind (NCT03490032) was a phase 1/2, open-label, multicenter study of administration of 3 MBq/kg of 68Ga-PSMA-R2 (from >150 to ≤250 MBq) in patients with biochemical recurrence (BCR) or metastatic PC (mPC). Participants underwent baseline conventional imaging (CT/MRI or bone scan) and PET/CT. Whole-body PET/CT imaging sequences were obtained between 20 min and 4 h after injection. Primary endpoints were safety and tolerability; secondary endpoints included biodistribution, potential lesion identification, pharmacokinetics, and dosimetry. Potential lesions were identified by 2 masked expert panels; a third panel evaluated the identified lesions. Results: Six patients with BCR were enrolled into phase 1, and 24 patients with BCR or mPC (n = 12 each) into phase 2. Thirteen treatment-emergent adverse events were reported, including 1 serious adverse event (ileus), unrelated to drug administration. All adverse events were mild or moderate and deemed not related to 68Ga-PSMA-R2. Peak blood concentration of 68Ga-PSMA-R2 was typically observed approximately 5 min after injection, steadily decreasing over 6 h. Mean absorbed radiation dose was highest in the urinary bladder wall (0.120 mGy/MBq) and kidney (0.061 mGy/MBq). No other organ mean absorbed radiation dose exceeded 0.020 mGy/MBq. Mean absorbed radiation doses in the salivary and lacrimal glands were 0.016 and 0.008 mGy/MBq, respectively. Mean total body absorbed radiation dose was 0.014 mGy/MBq. Mean effective total body dose was 0.015 mSv/MBq (range, 0.012-0.018 mSv/MBq). 68Ga-PSMA-R2 PET/CT detected 85 lesions in 22 participants at 1 h after injection and 103 lesions in 22 participants at 2 h after injection. Conventional imaging detected 49 lesions in 8 participants with mPC but none in participants with BCR. Conclusion: 68Ga-PSMA-R2 was well tolerated, with no drug-related treatment-emergent adverse events. Safety and preliminary imaging performance data support further development of 68Ga-PSMA-R2 as a diagnostic agent in patients with PC.
Keywords: 68Ga-PSMA-R2; PET; PSMA; dosimetry; prostate cancer.
© 2025 by the Society of Nuclear Medicine and Molecular Imaging.
Figures
References
-
- American Cancer Society. Survival rates for prostate cancer. https://www.cancer.org/cancer/types/prostate-cancer/detection-diagnosis-.... Accessed August 5, 2024.
-
- Wright GL, Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1:18–28. - PubMed
-
- Seifert R, Emmett L, Rowe SP, et al. . Second version of the prostate cancer molecular imaging standardized evaluation framework including response evaluation for clinical trials (PROMISE V2). Eur Urol. 2023;83:405–412. - PubMed
-
- Seifert R, Gafita A, Telli T, et al. . Standardized PSMA-PET imaging of advanced prostate cancer. Semin Nucl Med. 2024;54:60–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
