CORE-MD clinical risk score for regulatory evaluation of artificial intelligence-based medical device software
- PMID: 39915308
- PMCID: PMC11802784
- DOI: 10.1038/s41746-025-01459-8
CORE-MD clinical risk score for regulatory evaluation of artificial intelligence-based medical device software
Abstract
The European CORE-MD consortium (Coordinating Research and Evidence for Medical Devices) proposes a score for medical devices incorporating artificial intelligence or machine learning algorithms. Its domains are summarised as valid clinical association, technical performance, and clinical performance. High scores indicate that extensive clinical investigations should be undertaken before regulatory approval, whereas lower scores indicate devices for which less pre-market clinical evaluation may be balanced by more post-market evidence.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: F.R.: Occasional travel and accommodation costs to CORE–MD meetings were supported within the EU Horizon 2020 grant budget (2022-204); senior associate editor European Heart Journal, Cardiovascular Imaging. E.B.: external collaborative expert at the European Medicine Agency between 2023 and 2024 (unpaid collaboration); Occasional travel and accommodation costs to CORE–MD meetings were supported within the EU Horizon 2020 grant budget (2022-204). N.B.: Honorarium for presentation at Great Wall Cardiac Congress; ESC paid Article Processing Costs for one article in the European Heart Journal – Digital Health; Heart Rhythm Society paid travel costs for presentation at HRX; ESC Vice-chair Digital Health Committee; Editor-in-Chief of European Heart Journal – Digital Health. E.C.: Research contract with Luxottica S.p.A, Payment to my institution; Research contract with Croce Rossa Italiana, Payment to my institution; Project evaluator for the Autonomous Province of Trento, Italy; Honoraria for presentations from Dynamicom Education Srl, Summeet Srl, AIM Italy S.r.l.; Member of the Regulatory Affairs committee of the European Society of Cardiology. R.D.: minority shareholder in myocardium.AI; Consulting fees were paid directly to him. The company develops AI products for cardiac MR image analysis and is in the process of applying for regulatory approval. S.G.: German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) through the European Union-financed NextGenerationEU programme under grant number 16KISA100K, project PATH—‘Personal Mastery of Health and Wellness Data’. The European Commission under the Horizon Europe Programme, as part of the projects CYMEDSEC (101094218) and ASSESS-DHT (101137347). S.G. has or has had consulting relationships with Una Health GmbH, Lindus Health Ltd.; Flo Ltd, Thymia Ltd., FORUM Institut für Management GmbH, High-Tech Gründerfonds Management GmbH, Ada Health GmbH, and he holds share options in Ada Health GmbH. Support and honoraria for TK Health Economics Forum at MEDICA. Advisory Group member of the EY-coordinated ‘Study on Regulatory Governance and Innovation in the Field of Medical Devices’ conducted on behalf of the DG SANTE of the European Commission. Share options in Ada Health GmbH. S.G. is a News and Views Editor for npj Digital Medicine. S.G. played no role in the internal review or decision to publish this article. G.M.: Occasional travel and accommodation costs to CORE–MD meetings were supported within the EU Horizon 2020 grant budget (2022-204). Health Products Regulatory Authority, Source of employment. G.O.: Health Products Regulatory Authority, Source of employment. J.B.R.: none. B.V.: Berrow Foundation Lord Florey scholarship. A.G.: Chair, Regulatory Affairs Committee, Biomedical Alliance in Europe.
Figures
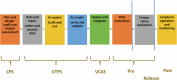



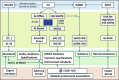
References
-
- Rajpurkar, P., Chen, E., Banerjee, O. & Topol, E. J. AI in health and medicine. Nat. Med.28, 31–38 (2022). - PubMed
-
- Hunter, D. J. & Holmes, C. Where medical statistics meets artificial intelligence. N. Engl. J. Med.389, 1211–1219 (2023). - PubMed
-
- World Health Organization. Regulatory considerations on artificial intelligence for health. https://www.who.int/publications/i/item/9789240078871 (2023).
-
- Ellahham, S., Ellahham, N. & Simsekler, M. C. E. Application of artificial intelligence in the health care safety context: opportunities and challenges. Am. J. Med. Qual.35, 341–348 (2020). - PubMed
Publication types
Grants and funding
- 945260/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945260/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945260/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945260/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945260/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945260/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945260/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945260/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945260/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945260/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945260/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945260/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
LinkOut - more resources
Full Text Sources

