A visual cortical-lateral posterior thalamic nucleus circuit regulates depressive-like behaviors in male mice
- PMID: 39915439
- PMCID: PMC11802872
- DOI: 10.1038/s41467-024-55600-4
A visual cortical-lateral posterior thalamic nucleus circuit regulates depressive-like behaviors in male mice
Abstract
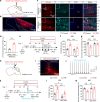
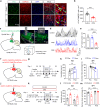
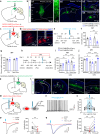
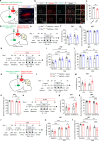
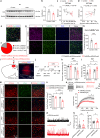
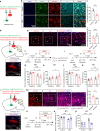
Depression, a prevalent psychiatric disorder of ambiguous etiology and high heterogeneity, has been recently linked to the primary visual cortex (V1). However, the precise circuits mediating the impact of V1 on depressive-like behaviors are poorly understood. Here, we demonstrate that the V1, specifically the lateral posterior nucleus of the thalamus (LP)-projecting V1 glutamatergic subpopulation (GluV1→LP neurons), shows reduced activity after chronic restraint stress (CRS) in male mice, leading to depressive-like behaviors. Optogenetic or chemogenetic activation of these neurons ameliorated depressive-like behaviors in CRS-depressed mice, whereas reducing activity exacerbated these behaviors. This reduction in GluV1→LP neurons activity was predominantly due to a decrease in the guanine nucleotide-binding protein subunit gamma-4 (Gγ4). Overexpression of Gγ4 in the GluV1→LP neurons produced antidepressant-like effects, suggesting that Gγ4 is a crucial regulator of mood. Collectively, these results reveal a V1→LP circuit that modulates depressive-like behaviors, suggesting potential targets for therapeutic interventions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

