Distinct immune cell infiltration patterns in pancreatic ductal adenocarcinoma (PDAC) exhibit divergent immune cell selection and immunosuppressive mechanisms
- PMID: 39915477
- PMCID: PMC11802853
- DOI: 10.1038/s41467-024-55424-2
Distinct immune cell infiltration patterns in pancreatic ductal adenocarcinoma (PDAC) exhibit divergent immune cell selection and immunosuppressive mechanisms
Abstract
Pancreatic ductal adenocarcinoma has a dismal prognosis. A comprehensive analysis of single-cell multi-omic data from matched tumour-infiltrated CD45+ cells and peripheral blood in 12 patients, and two published datasets, reveals a complex immune infiltrate. Patients have either a myeloid-enriched or adaptive-enriched tumour microenvironment. Adaptive immune cell-enriched is intrinsically linked with highly distinct B and T cell clonal selection, diversification, and differentiation. Using TCR data, we see the largest clonal expansions in CD8 effector memory, senescent cells, and highly activated regulatory T cells which are induced within the tumour from naïve cells. We identify pathways that potentially lead to a suppressive microenvironment, including investigational targets TIGIT/PVR and SIRPA/CD47. Analysis of patients from the APACT clinical trial shows that myeloid enrichment had a shorter overall survival compared to those with adaptive cell enrichment. Strategies for rationale therapeutic development in this disease include boosting of B cell responses, targeting immunosuppressive macrophages, and specific Treg cell depletion approaches.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.S. held a personal fellowship from BMS during this study with a grant provided to conduct experiments and also has research funding from Alchemab, has received speaker fees and travel grants from Astrazeneca, Novartis and Servier; and also conducts trials with Astrazeneca, Novartis, Roche, Genentech and BioNTech. E.A.B. is a contributor to intellectual property licensed by Oxford University Innovation to AstraZeneca and held a research award from Guts UK/Dr Falk during this project. D.J.R., M.W., S.C., T.L., P.V.S., and A.V.R. are current or former employees and shareholders of BMS. M.R.M. reports grants from GRAIL, Roche, Astrazeneca, BMS, Infinitopes, Immunocore, and study fees from BMS, Pfizer, MSD, Regeneron, BiolineRx, Replimune and Novartis outside of the submitted work. M.L.D. is on the SAB for Adaptimmune and Singula Bio, consults for Molecular Partners, Enara Bio, Labgenius and Astra Zeneca, and undertakes research supported by BMS, Cue Biopharma, Boehringer Ingelheim, Regeneron and Evolveimmune outside the submitted work. The remaining authors declare no competing interests. R.B.-R. is a co-founder of Alchemab Therapeutics Ltd and consultant for Alchemab Therapeutics Ltd, Roche, Enara Bio, UCB and GSK.
Figures

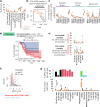
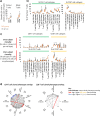


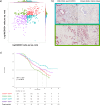
References
-
- Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin.73, 17–48 (2023). - PubMed
-
- Bockorny, B. et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat. Med.26, 878–885 (2020). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

