The anti-circumsporozoite antibody response to repeated, seasonal booster doses of the malaria vaccine RTS,S/AS01E
- PMID: 39915506
- PMCID: PMC11802723
- DOI: 10.1038/s41541-025-01078-0
The anti-circumsporozoite antibody response to repeated, seasonal booster doses of the malaria vaccine RTS,S/AS01E
Abstract
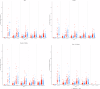
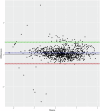
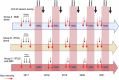
The recently deployed RTS,S/AS01E malaria vaccine induces a strong antibody response to the circumsporozoite protein (CSP) on the surface of the Plasmodium falciparum sporozoite which is associated with protection. The anti-CSP antibody titre falls rapidly after primary vaccination, associated with a decline in efficacy, but the antibody titre and the protective response can be partially restored by a booster dose of vaccine, but this response is also transitory. In many malaria- endemic areas of Africa, children are at risk of malaria, including severe malaria, until they are five years of age or older and to sustain protection from malaria for this period by vaccination with RTS,S/AS01E, repeated booster doses of vaccine may be required. However, there is little information about the immune response to repeated booster doses of RTS,S/AS01E. In many malaria-endemic areas of Africa, the burden of malaria is largely restricted to the rainy season and, therefore, a recent trial conducted in Burkina Faso and Mali explored the impact of repeated annual booster doses of RTS,S/AS01E given immediately prior to the malaria transmission season until children reached the age of five years. Anti-CSP antibody titres were measured in sera obtained from a randomly selected subset of children enrolled in this trial collected before and one month after three priming and four annual booster doses of vaccine using the GSK ELISA developed at the University of Ghent and, in a subset of these samples, by a multiplex assay developed at the University of Oxford. Three priming doses of RTS,S/AS01E induced a strong anti-CSP antibody response (GMT 368.9 IU/mL). Subsequent annual, seasonal booster doses induced a strong, but lower, antibody response; the GMT after the fourth booster was 128.5 IU/mL. Children whose antibody response was in the upper and middle terciles post vaccination had a lower incidence of malaria during the following year than children in the lowest tercile. Results obtained with GSK ELISA and the Oxford Multiplex assay were strongly correlated (Pearson's correlation coefficient, r = 0.94; 95% CI, 0.93-0.95). Although anti-CSP antibody titres declined after repeated booster doses of RTS,S/AS01E a high, although declining, level of efficacy was sustained suggesting that there may have been changes in the characteristics of the anti-CSP antibody following repeated booster doses.Clinical Trials Registration. NCT03143218.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: O.O. and K.E. are employees of the GSK group of companies and have restricted shares in the GSK group of companies. K.E. is a named inventor on patents relating to the R21/matrix M vaccine. A Dicko. reports a research grant from Grand Challenges Canada to test safety and efficacy of the PfSPZ vaccine for pregnant women and unborn children and a research contract with Oxford University to conduct a phase 3 randomized, controlled multicentre trial to evaluate the efficacy of the R21/Matrix-M vaccine in African children against clinical malaria outside of the submitted work. All other authors report no potential conflicts.
Figures


References
-
- WHO. WHO recommends ground breaking malaria vaccine for children at risk; Historic RTS, S/AS01 recommendation can reinvigorate the fight against malaria. https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-m... (2021).
-
- WHO. WHO recommends R21/Matrix-M vaccine for malaria prevention in updated advice on immunization. https://www.who.int/news/item/02-10-2023-who-recommends-r21-matrix-m-vac... (2023).
-
- Chandramohan, D. et al. Seasonal malaria vaccination with or without seasonal malaria chemoprevention. N. Engl. J. Med.385, 1005–1017 (2021). - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

