Effects of time restricted feeding combined with Lacto Ovo vegetarian diet on metabolic associated fatty liver disease management: a randomized clinical trial
- PMID: 39915600
- PMCID: PMC11803106
- DOI: 10.1038/s41598-025-88773-z
Effects of time restricted feeding combined with Lacto Ovo vegetarian diet on metabolic associated fatty liver disease management: a randomized clinical trial
Abstract
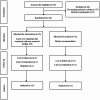
Metabolic Associated Fatty Liver Disease (MAFLD) is becoming a major global health concern due to its links with obesity, insulin resistance, and cardiovascular risk. This randomized clinical trial assessed the effects of combining time-restricted feeding (TRF; 16/8) with a Lacto-Ovo-Vegetarian (LOV) diet on various factors in overweight and obese patients with MAFLD. Forty-six participants were randomly assigned to either the intervention group (TRF with LOV diet) or the control group, with 21 participants completing the 12-week study in each group. The intervention group showed significant reductions in weight (-8.07 ± 4.31 kg), BMI (-2.70 ± 1.32 kg/m2), waist circumference (-8.00 ± 4.06 cm), as well as ALT (-17.14 ± 14.33 U/L), GGT (-21.09 ± 24.06 U/L), Fatty Liver Index (-26.90 ± 15.81), insulin levels (-3.89 ± 4.69 mU/L), and TNF-α (-11.85 ± 12.52 pg/mL) compared to the control group (all P < 0.05). Lipid profiles also improved with a reduction in triglycerides (-46.85 ± 54.55 mg/dL) and an increase in HDL-C (3.91 ± 5.07 mg/dL) in the intervention group compared to the control group (P < 0.05). These findings imply that TRF combined with a LOV diet enhances metabolic markers, liver health, and weight loss, thus potentially offering a practical dietary approach for managing MAFLD. Further long-term studies are necessary to validate these results and investigate their clinical applications.
Keywords: Lacto-ovo-vegetarian diet; MAFLD; NAFLD; Obesity; RCT; Time restricted feeding.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: The study protocol was approved by the Ethics Committee of the Faculty of Nutrition and Food Technology at Shahid Beheshti University of Medical Sciences in Tehran, Iran (Ethics committee reference number: IR.SBMU.NNFTRI.REC.1402.037) and all participants signed a written informed consent form. This study complies with the Declaration of Helsinki and was performed according to ethics committee approval.
References
-
- Beiriger, J. et al. Advancements in understanding and treating NAFLD: a Comprehensive Review of Metabolic-Associated fatty liver disease and emerging therapies. Livers3 (4), 637–656 (2023).
-
- Riazi, K. et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol.7 (9), 851–861 (2022). - PubMed
-
- Marjot, T., Moolla, A., Cobbold, J. F., Hodson, L. & Tomlinson, J. W. Nonalcoholic fatty liver disease in adults: current concepts in etiology, outcomes, and management. Endocr. Rev. ;41(1). (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous