Trichromacy is insufficient for mate detection in a mimetic butterfly
- PMID: 39915690
- PMCID: PMC11802900
- DOI: 10.1038/s42003-025-07472-7
Trichromacy is insufficient for mate detection in a mimetic butterfly
Abstract
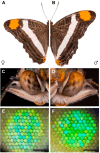
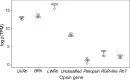
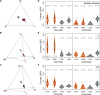
Color vision is thought to play a key role in the evolution of animal coloration, while achromatic vision is rarely considered as a mechanism for species recognition. Here we test the hypothesis that brightness vision rather than color vision helps Adelpha fessonia butterflies identify potential mates while their co-mimetic wing coloration is indiscriminable to avian predators. We examine the trichromatic visual system of A. fessonia and characterize its photoreceptors using RNA-seq, eyeshine, epi-microspectrophotometry, and optophysiology. We model the discriminability of its wing color patches in relation to those of its co-mimic, A. basiloides, through A. fessonia and avian eyes. Visual modeling suggests that neither A. fessonia nor avian predators can readily distinguish the co-mimics' coloration using chromatic or achromatic vision under natural conditions. These results suggest that mimetic colors are well-matched to visual systems to maintain mimicry, and that mate avoidance between these two look-alike species relies on other cues.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Prudic, K. L. & Khera, S. Isolation, identification, quantification potential defensive compounds in the viceroy butterfly and its larval host-plant, Carolina willow. J. Chem. Ecol.33, 1149–1159 (2007). - PubMed
-
- Rowland, H. M., Ihalainen, E., Lindström, L., Mappes, J. & Speed, M. P. Co-mimics have a mutualistic relationship despite unequal defences. Nature448, 64–67 (2007). - PubMed
-
- Thurman Tj Seymoure, B. M. A bird’s eye view of two mimetic tropical butterflies: coloration matches predator’s sensitivity. J. Zool.298, 159–168 (2016).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

