Live imaging of the extracellular matrix with a glycan-binding fluorophore
- PMID: 39915692
- PMCID: PMC12074998
- DOI: 10.1038/s41592-024-02590-2
Live imaging of the extracellular matrix with a glycan-binding fluorophore
Abstract
All multicellular systems produce and dynamically regulate extracellular matrices (ECMs) that play essential roles in both biochemical and mechanical signaling. Though the spatial arrangement of these extracellular assemblies is critical to their biological functions, visualization of ECM structure is challenging, in part because the biomolecules that compose the ECM are difficult to fluorescently label individually and collectively. Here, we present a cell-impermeable small-molecule fluorophore, termed Rhobo6, that turns on and red shifts upon reversible binding to glycans. Given that most ECM components are densely glycosylated, the dye enables wash-free visualization of ECM, in systems ranging from in vitro substrates to in vivo mouse mammary tumors. Relative to existing techniques, Rhobo6 provides a broad substrate profile, superior tissue penetration, non-perturbative labeling, and negligible photobleaching. This work establishes a straightforward method for imaging the distribution of ECM in live tissues and organisms, lowering barriers for investigation of extracellular biology.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.F., G.Y., W.K. and K.P. are authors on a patent application filed by the Howard Hughes Medical Institute relating to this work (US Application Serial No. 63/637,164). The remaining authors declare no competing interests.
Figures
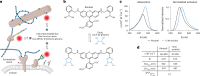


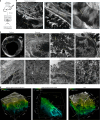




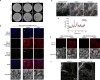




Update of
-
Imaging the extracellular matrix in live tissues and organisms with a glycan-binding fluorophore.bioRxiv [Preprint]. 2024 May 10:2024.05.09.593460. doi: 10.1101/2024.05.09.593460. bioRxiv. 2024. Update in: Nat Methods. 2025 May;22(5):1070-1080. doi: 10.1038/s41592-024-02590-2. PMID: 38766047 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

