Evaluation of dried blood spot sampling for real-time PCR malaria diagnostics in a rural setting in Angola
- PMID: 39915805
- PMCID: PMC11803990
- DOI: 10.1186/s13071-025-06685-3
Evaluation of dried blood spot sampling for real-time PCR malaria diagnostics in a rural setting in Angola
Abstract
Background: Malaria is the parasitic disease with the highest morbidity and mortality worldwide. Angola is one of the five sub-Saharan African countries with the highest malaria burden. Real-time PCR diagnosis in endemic areas has not been implemented due to its high cost and the need for adequate infrastructure. Dried blood spots (DBSs) are an alternative for collecting, preserving, and transporting blood samples to reference laboratories. The objective of the study was to assess the efficacy of DBS as a sampling method for malaria research studies employing real-time PCR.
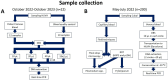
Methods: The study was divided into two phases: (i) prospective study at the Hospital Universitario Vall d'Hebron (HUVH) to compare real-time PCR from whole blood or DBS, including 12 venous blood samples from patients with positive real-time PCR for Plasmodium spp. and 10 quality control samples (nine infected samples and one negative control). Samples were collected as DBSs (10, 20, 50 µl/circle). Samples from both phases of the study were analyzed by generic real-time PCR (Plasmodium spp.) and the subsequent positive samples underwent species-specific real-time PCR (Plasmodium species) and (ii) cross-sectional study conducted at the Hospital Nossa Senhora da Paz, Cubal (Angola), including 200 participants with fever. For each patient, a fresh capillary blood specimen [for thin and thick blood films and rapid diagnostic test (RDT)] and venous blood, collected as DBSs (two 10-µl circles were combined for a total volume of 20 µl of DBS), were obtained. DBSs were sent to HUVH, Barcelona, Spain.
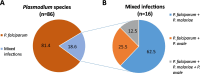
Results: (i) Real-time PCR from whole blood collection was positive for 100% of the 21 Plasmodium spp.-infected samples, whereas real-time PCR from DBSs detected Plasmodium spp. infection at lower proportions: 76.19% (16/21) for 10 µl, 85.71% (18/21) for 20 µl, 88.24% (15/17) for 50 µl and 85.71% (18/21) for 100 µl DBSs. (ii) Field diagnosis (microscopy and/or RDT) showed a 51.5% (103/200) positivity rate, while 50% (100/200) of the DBS samples tested positive by real-time PCR. Using field diagnosis as the reference method, the sensitivity of real-time PCR in DBS samples was 77.67% with a specificity of 79.38%. Plasmodium species were identified in 86 samples by real-time PCR: 81.40% (16/86) were caused by Plasmodium falciparum, 11.63% (10/86) were coinfections of P. falciparum + P. malariae, 4.65% (4/86) were P. falciparum + P. ovale, and 2.33% (2/86) were triple coinfections.
Conclusions: The DBS volume used for DNA extraction is a determining factor in the performance of real-time PCR for Plasmodium DNA detection. A DBS volume of 50-100 µl appears to be optimal for malaria diagnosis and Plasmodium species determination by real-time PCR. DBS is a suitable method for sample collection in Cubal followed by real-time PCR analysis in a reference laboratory.
Keywords: Diagnosis; Dried blood spots; Malaria; Real-time PCR; Sampling; Whole blood.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Vall d'Hebron University Hospital Drug Research Ethics Committee [PR(AG)79/2024] and by the Ethics Committee of the Angolan Ministry of Health (39 C.E/MINSA.INIS/2022). Each participant signed an informed consent form (in the case of minors, parental/guardian consent was obtained) after being informed of the objectives of the study. All procedures were performed according to the ethical guidelines of the 2013 Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Varo R, Chaccour C, Bassat Q. Update on malaria. Med Clin (Barc). 2020;155:395–402. - PubMed
-
- World Health Organization (WHO). World malaria report 2023. 2023. https://cdn.who.int/media/docs/default-source/malaria/world-malaria-repo.... Accessed 30 Sep 2024.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

