Heterogeneous clinical phenotypes of sporadic early-onset Alzheimer's disease: a neuropsychological data-driven approach
- PMID: 39915859
- PMCID: PMC11800584
- DOI: 10.1186/s13195-025-01689-8
Heterogeneous clinical phenotypes of sporadic early-onset Alzheimer's disease: a neuropsychological data-driven approach
Abstract
Background: The clinical presentations of early-onset Alzheimer's disease (EOAD) and late-onset Alzheimer's disease are distinct, with EOAD having a more aggressive disease course with greater heterogeneity. Recent publications from the Longitudinal Early-Onset Alzheimer's Disease Study (LEADS) described EOAD as predominantly amnestic, though this phenotypic description was based solely on clinical judgment. To better understand the phenotypic range of EOAD presentation, we applied a neuropsychological data-driven method to subtype the LEADS cohort.
Methods: Neuropsychological test performance from 169 amyloid-positive EOAD participants were analyzed. Education-corrected normative comparisons were made using a sample of 98 cognitively normal participants. Comparing the relative levels of impairment between each cognitive domain, we applied a cut-off of 1 SD below all other domain scores to indicate a phenotype of "predominant" impairment in a given cognitive domain. Individuals were otherwise considered to have multidomain impairment. Whole-cortex general linear modeling of cortical atrophy was applied as an MRI-based validation of these distinct clinical phenotypes.
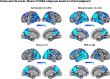
Results: We identified 6 phenotypic subtypes of EOAD: Dysexecutive Predominant (22% of sample), Amnestic Predominant (11%), Language Predominant (11%), Visuospatial Predominant (15%), Mixed Amnestic/Dysexecutive Predominant (11%), and Multidomain (30%). These phenotypes did not differ by age, sex, or years of education. The APOE ɛ4 genotype was enriched in the Amnestic Predominant group, who were also rated as least impaired. Cortical thickness analysis validated these clinical phenotypes with dissociations in atrophy patterns observed between the Dysexecutive and Amnestic Predominant groups. In contrast to the heterogeneity observed from our neuropsychological data-driven approach, diagnostic classifications for this same sample based solely on clinical judgment indicated that 82% of individuals were amnestic-predominant, 9% were non-amnestic, 4% met criteria for Posterior Cortical Atrophy, and 5% met criteria for Primary Progressive Aphasia.
Conclusion: A neuropsychological data-driven method to phenotype EOAD individuals uncovered a more detailed understanding of the presenting heterogeneity in this atypical AD sample compared to clinical judgment alone. Clinicians and patients may over-report memory dysfunction at the expense of non-memory symptoms. These findings have important implications for diagnostic accuracy and treatment considerations.
Keywords: Alzheimer’s disease; Clinical; Cognition; Early-onset; Neuropsychology; Phenotypes; Variants.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Institutional Review Board approval was obtained through a central review board overseen by Indiana University. Written informed consent was obtained from study participants or authorized representatives. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- van Vliet D, de Vugt ME, Bakker C, Pijnenburg YA, Vernooij-Dassen MJ, Koopmans RT, et al. Time to diagnosis in young-onset dementia as compared with late-onset dementia. Psychol Med. 2013;43(2):423–32. - PubMed
-
- Koedam EL, Pijnenburg YA, Deeg DJ, Baak MM, van der Vlies AE, Scheltens P, et al. Early-onset dementia is associated with higher mortality. Dement Geriatr Cogn Disord. 2008;26(2):147–52. - PubMed
-
- Smits LL, Pijnenburg YA, van der Vlies AE, Koedam EL, Bouwman FH, Reuling IE, et al. Early onset APOE E4-negative Alzheimer’s disease patients show faster cognitive decline on non-memory domains. Eur Neuropsychopharmacol. 2015;25(7):1010–7. - PubMed
-
- Joubert S, Gour N, Guedj E, Didic M, Gueriot C, Koric L, et al. Early-onset and late-onset Alzheimer’s disease are associated with distinct patterns of memory impairment. Cortex. 2016;74:217–32. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

