Caloric Restriction Preserves BBB Integrity After Transient Focal Cerebral Ischemia Through Reducing Neutrophil Infiltration
- PMID: 39915908
- PMCID: PMC11802461
- DOI: 10.1111/cns.70257
Caloric Restriction Preserves BBB Integrity After Transient Focal Cerebral Ischemia Through Reducing Neutrophil Infiltration
Abstract
Aims: Caloric restriction is a health-promoting lifestyle that has been reported to protect both white and gray matter in cases of ischemic stroke. This study will explore the underlying mechanism of restricted feeding (RF) and provide a theoretical basis for precise clinical treatment of stroke.
Methods: In this study, we pretreated C57BL/6J mice with 70% RF for a continuous 28-day period prior to 60 min of transient focal cerebral ischemia (tFCI). Histological staining, diffusion tensor imaging (DTI), and behavioral assessments were used to assess RF's neuroprotection following tFCI. Immunofluorescence staining, quantitative real-time PCR, and flow cytometry were conducted to evaluate brain inflammation post-tFCI. Western blot, immunofluorescence staining, tracers, and electric microscopy were used to assess the blood-brain barrier (BBB) integrity. Peripheral neutrophils were cleared by administrating an anti-Ly6G antibody.
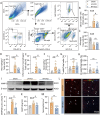
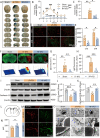
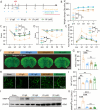
Results: Initially, DTI, NeuN staining, and a batch of behavioral tests verified that RF significantly mitigated both gray/white matter injury and neurological deficits in the short- and long-term following tFCI. RF mice showed more anti-inflammatory microglia in their brains, along with reduced inflammatory cytokines, and chemokines. Interestingly, RF significantly reduced the neutrophils and macrophage infiltration. Subsequently, we observed that RF mice exhibited better BBB integrity following tFCI, with reduced neutrophil infiltration and matrix metalloprotein-9 release. Furthermore, the clearance of neutrophils with anti-Ly6G antibody in ad libitum feeding mice (LF-Ly6G) elicited comparable neuroprotective effects to those observed in RF, including improvements in neurological deficits, reductions in infarct volume, and mitigation of BBB damage.
Conclusions: In summary, our findings suggest that RF maintains the BBB integrity following ischemic stroke at least partially by reducing neutrophil infiltration, thereby alleviating both neurological and histological impairments.
Keywords: blood–brain barrier; caloric restriction; neutrophils; stroke.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- “World Stroke Day 2022,” accessed September 2, 2024, https://www.who.int/srilanka/news/detail/29‐10‐2022‐world‐stroke‐day‐2022.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

