Thoracic epidural analgesia vs. patient-controlled intravenous analgesia for patients undergoing open or laparoscopic colorectal cancer surgery: An observational study
- PMID: 39916756
- PMCID: PMC11783615
- DOI: 10.1097/EA9.0000000000000013
Thoracic epidural analgesia vs. patient-controlled intravenous analgesia for patients undergoing open or laparoscopic colorectal cancer surgery: An observational study
Abstract
Background: Thoracic epidural analgesia (TEA) is an invasive technique with potential side effects but is widely used in enhanced recovery after surgery (ERAS) programmes in colorectal cancer surgery. The effects of TEA on postoperative length of hospital stay (LOS) or morbidity is still debated.
Objectives: The main objective was to evaluate the postoperative analgesic effectiveness of TEA compared with patient-controlled intravenous analgesia (PCIA) after open or laparoscopic colorectal surgery, and whether TEA contributes to enhanced recovery.
Design: A retrospective single-centre, observational study.
Setting: Dutch tertiary-care university hospital.
Patients: All consecutive adult patients undergoing colorectal cancer surgery from 1 January 2014 to 31 December 2016, with ASA status I-IV, were included. Exclusion criteria were hypersensitivity to opioid or local anaesthetic substances, or the use of multiple secondary anaesthetic techniques.
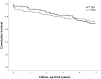
Main outcome measures: The primary outcome, postoperative pain assessed with a Numeric Rating Scale on postoperative days 1 to 3 inclusive. Secondary endpoints were LOS, the incidence of epidural related side effects, major complications and the 5-year survival rate. Using linear mixed models, pain scores were compared between patients who received TEA and PCIA.
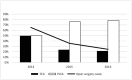
Results: Of 422 enrolled patients, 110 (32%) received TEA and 234 (68%) PCIA. Patients in the TEA group had lower pain scores: estimated NRS difference at rest; -0.79; 95% CI, -1.1 to -0.49; P < 0.001 and during movement -1.06; 95% CI, -1.39 to -0.73; P < 0.001. LOS, 30-day complication rate and overall survival at 5 years did not differ between the groups.
Conclusions: TEA in open or laparoscopic colorectal surgery is associated with moderately better postoperative pain control but does not affect LOS, postoperative morbidity, mortality nor long-term survival. The current clinical indication for TEA in colorectal surgery remains unchanged.
Trial registration: International clinical trial registration number: ISRCTN11426678; retrospectively registered 26 February 2021.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Society of Anaesthesiology and Intensive Care.
Conflict of interest statement
Conflict of interest: none.
Figures
References
-
- Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 2019; 43:659–695. - PubMed
-
- Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009; 144:961–969. - PubMed
-
- Miller TE, Thacker JK, White WD, et al. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg 2014; 118:1052–1061. - PubMed
-
- Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet 2002; 359:1276–1282. - PubMed
LinkOut - more resources
Full Text Sources