Real-world assessment of effectiveness and safety of filgotinib in 286 patients with ulcerative colitis in 9 UK centres
- PMID: 39917051
- PMCID: PMC11798541
- DOI: 10.7573/dic.2024-11-1
Real-world assessment of effectiveness and safety of filgotinib in 286 patients with ulcerative colitis in 9 UK centres
Abstract
Background: Filgotinib, an oral Janus kinase 1 preferential inhibitor, has been shown to be an effective treatment for ulcerative colitis (UC) in pre-registration studies. We aimed to describe the treatment population, effectiveness and safety of filgotinib in a real-world cohort of patients with UC.
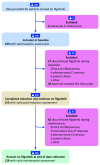
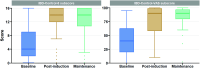
Methods: A retrospective observational cohort evaluation was conducted across nine UK inflammatory bowel disease centres. Baseline demographic and clinical data, clinical disease activity scores, endoscopic activity indices, and biomarkers (C-reactive protein and faecal calprotectin) were collected at baseline, at 8-12 weeks after initiation (post-induction) and during maintenance (the most recent review) where available. Effectiveness outcomes were assessed in patients with combined clinical disease activity and objective evidence of inflammation at filgotinib initiation.
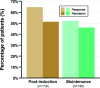
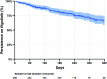
Results: Data were analysed for a total of 286 patients with a median follow-up time of 229 (IQR 113-324) days. The median age at filgotinib initiation was 38 (IQR 27-51) years, 64% were men and median disease duration was 5.1 (IQR 1.9-10.5) years; 56% had previous exposure to advanced therapies (biologics and small molecule) and 6% previously received tofacitinib. At the post-induction review, clinical response and remission were achieved in 65% and 51% of patients, respectively. There was a reduction in biomarkers and 78% of patients using corticosteroids at baseline were steroid-free. Persistence on filgotinib at 12 months was 66%. Adverse events were recorded in 30 patients with 8 patients discontinuing filgotinib as a result of an adverse event.
Conclusions: In a large real-world cohort of patients with UC, filgotinib appears to be effective and well-tolerated.
Keywords: colitis; filgotinib; quality of life; ulcerative colitis.
Copyright © 2025 Young D, Rahmany S, Taylor D, Davis E, Colwill M, Kalyanji Mehta S, Campbell R, Hazel K, Sethi-Arora K, Ritchie S, Heinson AI, Moyses H, Bodger K, Johnston E, Hicks L, Dhar A, Limdi J, Cooney R, Seenan JP, Patel K, Walsh A, Cummings F.
Conflict of interest statement
Disclosure and potential conflicts of interest: DY: payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Galapagos Biotech Limited, Takeda UK Ltd, Bristol-Myers Squibb; support for attending meetings and/or travel: Galapagos Biotech Limited, Tillotts Pharma UK. SR: consulting fees: Takeda; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Abbvie, Celltrion; support for attending meetings and/or travel: Celltrion. ED: payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Ferring Pharmaceuticals. MC: payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Dr Falk Pharma; support for attending meetings and/or travel: Ferring Pharmaceuticals; participation on a Data Safety Monitoring Board or Advisory Board: Dr Falk Pharma; receipt of equipment, materials, drugs, medical writing, gifts or other services: Pulse Today. SKM: payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Dr Falk Pharma; support for attending meetings and/or travel: Celltrion Healthcare. KS-A: payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Elsevier, Janssen, Ferring, Galapagos, Takeda, Celltrion; support for attending meetings and/or travel: Takeda, Janssen. KB: payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Takeda Ltd. LH: support for attending meetings and/or travel: Takeda. EJ: payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Dr Falk Pharma. AD: consulting fees: Dr Falk Pharma UK, Bristol-Myers Squibb, Takeda UK, Johnson and Johnson, Boston Scientific Corporation; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Takeda, BMS, Alfasigma, Abbvie, Tillotts UK, Dr Falk Pharma, Johnson and Johnson, Lilly; support for attending meetings and/or travel: Johnson and Johnson, Takeda, Abbvie, Tillotts Pharma, Synmed UK; participation on a Data Safety Monitoring Board or Advisory Board: Johnson and Johnson, Abbvie, Dr Falk Pharma, Tillotts UK. JL: grants or contracts from any entity: Galapagos; consulting fees: Abbvie, Janssen, Takeda, Pfizer, Bristol-Myers Squibb, Celltrion, Eli Lilly, Ferring; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Abbvie, Janssen, Takeda, Pfizer, Bristol-Myers Squibb, Celltrion, Eli Lilly, Ferring; support for attending meetings and/or travel: Janssen, Takeda, Pfizer; participation on a Data Safety Monitoring Board or Advisory Board: Janssen, Abbvie, Celltrion, Pfizer, Galapagos. JL is an Associate Editor for Drugs in Context. RC: consulting fees: speakers/research support/consulting fees from Dr Falk Pharma, Pfizer, Janssen-Cilag, Abbvie, Galapagos, Celltrion, Takeda; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: speakers/research support/consulting fees from Dr Falk Pharma, Pfizer, Janssen-Cilag, Abbvie, Galapagos, Celltrion, Takeda; other financial or non-financial interests: speakers/research support/consulting fees from Dr Falk Pharma, Pfizer, Janssen-Cilag, Abbvie, Galapagos, Celltrion and Takeda. JPS: payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Fresenius Kabi Limited, AbbVie, Tillotts Pharma UK, Janssen-Cilag, Pharmacosmos UK Ltd, Takeda UK Ltd, Bristol-Myers Squibb; support for attending meetings and/or travel: AbbVie, Tillotts Pharma UK, Janssen-Cilag; participation on a Data Safety Monitoring Board or Advisory Board: Galapagos NV, Dr Falk Pharma (UK), AbbVie. KP: consulting fees: speaker fees, consulting fees and/or education support from Abbvie, Dr Falk Pharma, Janssen, Takeda, Pfizer, PredictImmune, Celltrion, Galapagos; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: speaker fees, consulting fees and/or education support from Abbvie, Dr Falk Pharma, Janssen, Takeda, Pfizer, PredictImmune, Celltrion, Galapagos; support for attending meetings and/or travel: speaker fees, consulting fees and/or education support from Abbvie, Dr Falk Pharma, Janssen, Takeda, Pfizer, PredictImmune, Celltrion and Galapagos. AW: grants or contracts from AbbVie, Buhlmann, Dr Falk Pharma, Ferring, Galapagos, Helmsley trust, Janssen, Lilly, Pfizer, Takeda, Vifor, Norman Collisson Foundation; consulting fees from AbbVie, Buhlmann, Dr Falk Pharma, Ferring, Galapagos, Helmsley trust, Janssen, Lilly, Pfizer, Takeda, Vifor, Norman Collisson Foundation; other financial or non-financial interests from AbbVie, Buhlmann, Dr Falk Pharma, Ferring, Galapagos, Helmsley trust, Janssen, Lilly, Pfizer, Takeda, Vifor, Norman Collisson Foundation. FC: consulting fees from AbbVie, Amgen, BMS, Celltrion, Dr Falk Pharma, Ferring, Galapagos, Janssen, MSD, Napp Pharmaceuticals, Pfizer, Pharmacosmos, Sandoz, Biogen, Samsung, Takeda; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie, Amgen, BMS, Celltrion, Dr Falk Pharma, Ferring, Galapagos, Janssen, MSD, Napp Pharmaceuticals, Pfizer, Pharmacosmos, Sandoz, Biogen, Samsung, Takeda; participation on a Data Safety Monitoring Board or Advisory Board for AbbVie, Amgen, BMS, Celltrion, Dr Falk Pharma, Ferring, Galapagos, Janssen, MSD, Napp Pharmaceuticals, Pfizer, Pharmacosmos, Sandoz, Biogen, Samsung, Takeda; other financial or non-financial interests: research funding from Biogen, Amgen, Celltrion, Hospira/Pfizer, Janssen, Takeda, GSK, AZ. DT, RC, KH, SR, AIH and HM: No conflicts of interest to report. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2025/01/dic.2024-11-1-COI.pdf
Figures
References
-
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi: 10.1053/j.gastro.2020.12.031. - DOI - PubMed
-
- Medicines and Healthcare products Regulatory Agency. Janus kinase (JAK) inhibitors: new measures to reduce risks of major cardiovascular events, malignancy, venous thromboembolism, serious infections and increased mortality. Drug Safety Update. 2023;16(9):2.
LinkOut - more resources
Full Text Sources
Research Materials

