Ezh2 Shapes T Cell Plasticity to Drive Atherosclerosis
- PMID: 39917842
- PMCID: PMC12063685
- DOI: 10.1161/CIRCULATIONAHA.124.072384
Ezh2 Shapes T Cell Plasticity to Drive Atherosclerosis
Abstract
Background: The activation and polarization of T cells play a crucial role in atherosclerosis and dictate athero-inflammation. The epigenetic enzyme EZH2 (enhancer of zeste homolog 2) mediates the H3K27me3 (trimethylation of histone H3 lysine 27) and is pivotal in controlling T cell responses.
Methods: To detail the role of T cell EZH2 in atherosclerosis, we used human carotid endarterectomy specimens to reveal plaque expression and geography of EZH2. Atherosclerosis-prone Apoe (apolipoprotein E)-deficient mice with CD (cluster of differentiation) 4+ or CD8+ T cell-specific Ezh2 deletion (Ezh2cd4-knockout [KO], Ezh2cd8-KO) were analyzed to unravel the role of T cell Ezh2 in atherosclerosis and T cell-associated immune status.
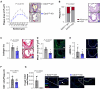
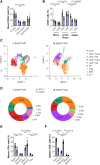
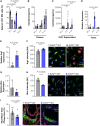
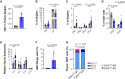
Results: EZH2 expression is elevated in advanced human atherosclerotic plaques and primarily expressed in the T cell nucleus, suggesting the importance of canonical EZH2 function in atherosclerosis. Ezh2cd4-KO, but not Ezh2cd8-KO, mice showed reduced atherosclerosis with fewer advanced plaques, which contained less collagen and macrophages, indicating that Ezh2 in CD4+ T cells drives atherosclerosis. In-depth analysis of CD4+ T cells of Ezh2cd4-KO mice revealed that absence of Ezh2 results in a type 2 immune response with increased Il-4 (interleukin 4) gene and protein expression in the aorta and lymphoid organs. In vitro, Ezh2-deficient T cells polarized macrophages toward an anti-inflammatory phenotype. Single-cell RNA-sequencing of splenic T cells revealed that Ezh2 deficiency reduced naive, Ccl5+ (C-C motif chemokine ligand 5) and regulatory T cell populations and increased the frequencies of memory T cells and invariant natural killer T (iNKT) cells. Flow cytometric analysis identified a shift toward Th2 (type 2 T helper) effector CD4+ T cells in Ezh2cd4-KO mice and confirmed a profound increase in splenic iNKT cells with increased expression of Plzf (promyelocytic leukemia zinc finger), which is the characteristic marker of the iNKT2 subset. Likewise, Zbtb16 ([zinc finger and BTB domain containing 16], the Plzf-encoding gene) transcripts were elevated in the aorta of Ezh2cd4-KO mice, suggesting an accumulation of iNKT2 cells in the plaque. H3K27me3-chromatin immunoprecipitation followed by quantitative polymerase chain reaction showed that T cell-Ezh2 regulates the transcription of the Il-4 and Zbtb16 genes.
Conclusions: Our study uncovers the importance of T cell EZH2 in human and mouse atherosclerosis. Inhibition of Ezh2 in CD4+ T cells drives type 2 immune responses, resulting in an accumulation of iNKT2 and Th2 cells, memory T cells and anti-inflammatory macrophages that limit the progression of atherosclerosis.
Keywords: EZH2; T-lymphocytes; atherosclerosis; epigenomics; natural killer T cells.
Conflict of interest statement
None.
Figures



Comment in
-
Cell Type-Level Epigenetics at the Frontier of Atherosclerosis Research.Circulation. 2025 May 13;151(19):1409-1411. doi: 10.1161/CIRCULATIONAHA.125.074275. Epub 2025 May 12. Circulation. 2025. PMID: 40354452 No abstract available.
References
-
- Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524–533. doi: 10.1038/s41586-021-03392-8 - PubMed
-
- Weber C, Habenicht AJR, von Hundelshausen P. Novel mechanisms and therapeutic targets in atherosclerosis: inflammation and beyond. Eur Heart J. 2023;44:2672–2681. doi: 10.1093/eurheartj/ehad304 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

