Efficacy and safety of laquinimod versus placebo in relapsing-remitting multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials
- PMID: 39917858
- PMCID: PMC11806468
- DOI: 10.1177/03000605241311437
Efficacy and safety of laquinimod versus placebo in relapsing-remitting multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials
Abstract
Objective: To investigate the safety and efficacy of laquinimod in treating relapsing-remitting multiple sclerosis (RRMS).
Methods: An extensive electronic search was conducted across PubMed, Embase, Cochrane Database of Systematic Reviews, and ClinicalTrials.gov to identify suitable studies. Risk of bias was assessed using Cochrane's Risk of Bias tool. Statistical analysis was performed using RevMan 5.4.1.
Results: The meta-analysis of four randomized controlled trials including 3665 patients found that laquinimod significantly reduced the annualized relapse rate compared with placebo (mean difference = -0.08, 95% confidence interval [CI] = -0.12, -0.04, I2 = 0%). For disability progression confirmed at 3 months, laquinimod provided a significant advantage over placebo (hazard ratio [HR] = 0.75, 95% CI = 0.59, 0.96, I2 = 25%), whereas no benefit was achieved at 6 months (HR = 0.69, 95% CI = 0.45, 1.06, I2 = 66%). Laquinimod was also significantly better than placebo in maintaining a relapse-free status (risk ratio [RR] = 1.14 95% CI = 1.06, 1.22, I2 = 10%). Laquinimod had a comparable safety profile as placebo (RR = 1.06, 95% CI = 0.81, 1.39, I2 = 33%).
Conclusions: These findings support the efficacy of laquinimod in managing RRMS but necessitate careful monitoring during treatment.
Keywords: Multiple sclerosis; adverse event; disability; disease-modifying therapy; laquinimod; meta-analysis; randomized controlled trial; relapsing-remitting; secondary progressive multiple sclerosis.
Conflict of interest statement
Declaration of conflicting interestThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


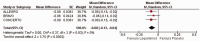
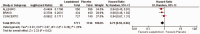
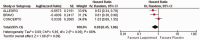
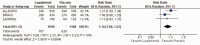
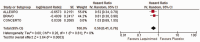

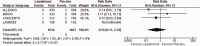
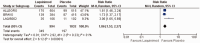
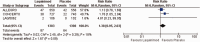
References
-
- Marcus R. What Is Multiple Sclerosis? Jama 2022; 328: 2078. DOI: 10.1001/jama.2022.14236. - PubMed
-
- The Multiple Sclerosis International Federation Atlas of MS, 3rd ed, September, 2020, https://www.atlasofms.org.
-
- Rae-Grant A, Day GS, Marrie RA, et al.. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018; 90: 777–788. DOI: 10.1212/wnl.0000000000005347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

