Phase I Study of the Safety, Tolerability, and Pharmacokinetics of Inhaled Voriconazole in Healthy Volunteers and Subjects With Stable Asthma
- PMID: 39918069
- PMCID: PMC11803457
- DOI: 10.1002/prp2.70064
Phase I Study of the Safety, Tolerability, and Pharmacokinetics of Inhaled Voriconazole in Healthy Volunteers and Subjects With Stable Asthma
Abstract
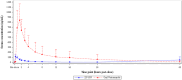
The aim of this study was to evaluate safety, tolerability, and pharmacokinetics (PK) of single and multiple doses of a novel inhaled formulation of voriconazole (ZP-059). In the single ascending dose part, 4 cohorts of 6 healthy subjects received one dose of inhaled voriconazole (5-40 mg). In the multiple ascending dose part, 3 cohorts of 6 subjects with mild asthma received voriconazole 10 mg twice daily [BID], 20 mg BID or 40 mg once daily. In the 2-period crossover part, 16 subjects with mild to moderate asthma each received one dose of inhaled voriconazole 20 mg and one dose of oral voriconazole 200 mg. A bioanalytical method was developed and validated to simultaneously determine concentrations of voriconazole and its metabolite N-oxide voriconazole in serum and sputum. Inhaled voriconazole was well tolerated with no treatment emergent adverse events (TEAEs) leading to treatment discontinuation. The PK profile of inhaled voriconazole showed rapid absorption, apparent greater than proportional increase in exposure with increasing dose, a consistent half-life across dosing, and large clearance and volume of distribution. Following repeat administration limited accumulation was observed. Systemic exposure following inhaled voriconazole was much lower than following oral voriconazole. Serum data confirmed that voriconazole was extensively metabolized also when administered by inhalation. Sputum data following inhaled voriconazole were limited but demonstrated increasing exposure with increasing dose. The current study shows the newly developed dry powder inhaled formulation of voriconazole to be safe and well tolerated, providing a possible improved treatment approach for patients affected by allergic bronchopulmonary aspergillosis. Trial Registration: ClinicalTrials.gov ID: NCT04229303.
Keywords: inhaled; pharmacokinetics; phase I; safety and tolerability; voriconazole.
© 2025 Zambon S.P.A. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
A. Cervetti, D. Colombo, F. Sala, E. Tiberio are current employees of Zambon. G. Caponetti is the inventor of the patents WO2022/1223009 “Method for manufacturing an inhalable powder comprising Voriconazole” and “Inhalable powder comprising Voriconazole in crystalline form” WO2022/123029. D. Singh has received consultancy fees from Aerogen, AstraZeneca, BIAL, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, EpiEndo, Genentech, GlaxoSmithKline, Glenmark, Gossamer Bio, Kinaset Therapeutics, Menarini, Novartis, Orion, Pulmatrix, Sanofi, Teva, Theravance Biopharma, and Verona Pharma.
Figures

References
-
- Kosmidis C. and Denning D. W., “The Clinical Spectrum of Pulmonary Aspergillosis,” Thorax 70 (2015): 270–277. - PubMed
-
- Sehgal I. S., Choudhary H., Dhooria S., et al., “Prevalence of Sensitization to Aspergillus Flavus in Patients With Allergic Bronchopulmonary Aspergillosis,” Medical Mycology 57, no. 3 (2019): 270–276. - PubMed
-
- Agarwal R., Aggarwal A. N., Gupta D., and Jindal S. K., “Aspergillus Hypersensitivity and Allergic Bronchopulmonary Aspergillosis in Patients With Bronchial Asthma: Systematic Review and Meta‐Analysis,” International Journal of Tuberculosis and Lung Disease 13, no. 8 (2009): 936–944. - PubMed
-
- Sehgal I. S., Saxena P., Dhooria S., et al., “Is the Prevalence of Allergic Bronchopulmonary Aspergillosis Greater in Severe Asthma?,” Journal of Allergy and Clinical Immunology: In Practice S2213‐2198, no. 24 (2024): 00890. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

