Selective STING Activation in Intratumoral Myeloid Cells via CCR2-Directed Antibody-Drug Conjugate TAK-500
- PMID: 39918395
- PMCID: PMC12046323
- DOI: 10.1158/2326-6066.CIR-24-0103
Selective STING Activation in Intratumoral Myeloid Cells via CCR2-Directed Antibody-Drug Conjugate TAK-500
Abstract
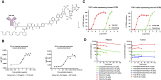
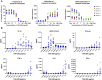
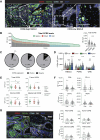
The tumor microenvironment in solid tumors contains myeloid cells that modulate local immune activity. Stimulator of IFN gene (STING) signaling activation in these myeloid cells enhances local type-I IFN production, inducing an innate immune response that mobilizes adaptive immunity and reprograms immunosuppressive myeloid populations to drive antitumor immunity. In this study, we generated TAK-500, an immune cell-directed antibody-drug conjugate, to deliver a STING agonist to CCR2+ human cells and drive enhanced antitumor activity relative to nontargeted STING agonists. Preclinically, TAK-500 triggered dose-dependent innate immune activation in vitro. In addition, a murine TAK-500 immune cell-directed antibody-drug conjugate surrogate enhanced innate and adaptive immune responses both in in vitro and murine tumor models. Spatially resolved analysis of CCR2 and immune cell markers in the tumor microenvironment of >1,000 primary human tumors showed that the CCR2 protein was predominantly expressed in intratumoral myeloid cells. Collectively, these data highlight the clinical potential of delivering a STING agonist to CCR2+ cells.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
V.A. Appleman, M.L. Ganno, E. Rosentrater, A.E. Maldonado Lopez, M.Y. Lee, C.I. Wang, L. Dong, T. Yoneyama, K.I. Piatkov, R.C. Gregory, A. Parent, N. Lineberry, C. Arendt, and A.O. Abu-Yousif report employment and ownership of stocks/shares with Takeda. A. Matsuda, D.M. Zhang, and J. Huang report employment with Takeda. S.A. Merrigan reports other support from Pfizer outside the submitted work. H.M. Lee reports a patent for WO2020229982 pending and a patent for WO2022097117 pending. L. Dong reports holding stocks of Takeda directly or indirectly through mutual funds during the conduct of the study. T. Yoneyama reports personal fees from Takeda Development Center Americas outside the submitted work. K.I. Piatkov reports personal fees from Takeda Pharmaceuticals International Co. and other support from Takeda Pharmaceuticals International Co. outside the submitted work. R.C. Gregory reports employment with Takeda during the conduct of the study. A. Parent reports personal fees from Takeda Pharmaceuticals during the conduct of the study. N. Lineberry reports other support from Takeda Pharmaceuticals during the conduct of the study, as well as other support from Takeda Pharmaceuticals outside the submitted work. K.A. Schalper reports grants from Takeda and personal fees from Takeda during the conduct of the study, as well as personal fees from Clinica Alemana Santiago, Shattuck Labs, AstraZeneca, EMD Serono, Takeda, Torque/Repertoire Therapeutics, CSR Life Sciences, Agenus, Genmab, OnCusp, Sanofi, Parthenon Therapeutics, Bristol Myers Squibb, Roche, Molecular Templates, Merck, Dynamicure, Indaptus Therapeutics, Moderna Inc., Merus, PeerView, Physicians Education Resource, and Forefront Collaborative and grants from TESARO/GSK, Takeda, Surface Oncology, Merck, Bristol Myers Squibb, AstraZeneca, Ribon Therapeutics, Eli Lilly and Company, Boehringer Ingelheim, Roche, Akoya Biosciences, and NextPoint Therapeutics outside the submitted work. A.O. Abu-Yousif reports personal fees and other support from Takeda during the conduct of the study. No disclosures were reported by the other authors.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

