Gene Variant Spectrum in Probands With Familial Exudative Vitreoretinopathy Using an Expanded Panel
- PMID: 39918476
- PMCID: PMC11809447
- DOI: 10.1167/iovs.66.2.23
Gene Variant Spectrum in Probands With Familial Exudative Vitreoretinopathy Using an Expanded Panel
Abstract
Purpose: To investigate the gene variant spectrum in patients with familial exudative vitreoretinopathy (FEVR).
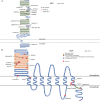
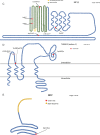
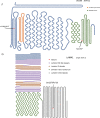
Methods: Probands clinically diagnosed with FEVR and their relatives were enrolled and clinical information and DNA collected. An expanded FEVR panel was used, including six recognized FEVR genes (FZD4, NDP, LRP5, TSPAN12, ZNF408, and CTNNB1) and 19 genes previously associated with ocular features overlapping FEVR (FEVR-associated genes). Variants identified using targeted next-generation sequencing and/or Sanger sequencing were analyzed and classified using the American College of Medical Genetics and Clinical Genome Resource Sequence Variant Interpretation (ClinGen SVI) working group recommendations to detect disease-causing variants (DCVs).
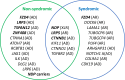
Results: Analyses of data from a cohort of 94 probands provided a molecular diagnosis for 39 (41.5%) probands: 34 (87.2%) had a single DCV, whereas 5 (12.8%) harbored more than 1 DCV. Of 41 total DCVs in solved probands, 33 (80.5%) were in 4 of the 6 recognized genes, LRP5, FZD4, TSPAN12, and NDP, whereas 8 were found in FEVR-associated genes, 6 in KIF11, and 2 (LAMA1 and DOCK6) each in association with a KIF11 DCV. Reanalyzing variants using the latest criteria impacted the variant classification in five probands (5.3%), changing variants that were once deemed likely pathogenic to variants of uncertain significance.
Conclusions: The expanded FEVR gene panel detected DCVs in nearly one-half of our cohort. Including the criteria used in classification will improve transparency of variant calls as more data become available. Four FEVR genes account for most cases, and the role of rare FEVR genes and candidate genes requires further study.
Conflict of interest statement
Disclosure:
Figures

References
-
- Robitaille J, MacDonald ML, Kaykas A, et al. .. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet. 2002; 32: 326–330. - PubMed
-
- Robitaille JM, Zheng B, Wallace K, et al. .. The role of Frizzled-4 mutations in familial exudative vitreoretinopathy and Coats disease. Br J Ophthalmol. 2011; 95: 574–579. - PubMed
-
- Criswick VG, Schepens CL.. Familial exudative vitreoretinopathy. Am J Ophthalmol. 1969; 68: 578–594. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

