Pediatric Gastrointestinal Tract Outcomes During the Postacute Phase of COVID-19
- PMID: 39918822
- PMCID: PMC11806396
- DOI: 10.1001/jamanetworkopen.2024.58366
Pediatric Gastrointestinal Tract Outcomes During the Postacute Phase of COVID-19
Abstract
Importance: The profile of gastrointestinal (GI) tract outcomes associated with the postacute and chronic phases of COVID-19 in children and adolescents remains unclear.
Objective: To investigate the risks of GI tract symptoms and disorders during the postacute (28-179 days after documented SARS-CoV-2 infection) and the chronic (180-729 days after documented SARS-CoV-2 infection) phases of COVID-19 in the pediatric population.
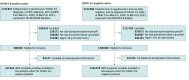
Design, setting, and participants: This retrospective cohort study was performed from March 1, 2020, to September 1, 2023, at 29 US health care institutions. Participants included pediatric patients 18 years or younger with at least 6 months of follow-up. Data analysis was conducted from November 1, 2023, to February 29, 2024.
Exposures: Presence or absence of documented SARS-CoV-2 infection. Documented SARS-CoV-2 infection included positive results of polymerase chain reaction analysis, serological tests, or antigen tests for SARS-CoV-2 or diagnosis codes for COVID-19 and postacute sequelae of SARS-CoV-2.
Main outcomes and measures: GI tract symptoms and disorders were identified by diagnostic codes in the postacute and chronic phases following documented SARS-CoV-2 infection. The adjusted risk ratios (ARRs) and 95% CI were determined using a stratified Poisson regression model, with strata computed based on the propensity score.
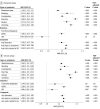
Results: The cohort consisted of 1 576 933 pediatric patients (mean [SD] age, 7.3 [5.7] years; 820 315 [52.0%] male). Of these, 413 455 patients had documented SARS-CoV-2 infection and 1 163 478 did not; 157 800 (13.6%) of those without documented SARS-CoV-2 infection had a complex chronic condition per the Pediatric Medical Complexity Algorithm. Patients with a documented SARS-CoV-2 infection had an increased risk of developing at least 1 GI tract symptom or disorder in both the postacute (8.64% vs 6.85%; ARR, 1.25; 95% CI, 1.24-1.27) and chronic (12.60% vs 9.47%; ARR, 1.28; 95% CI, 1.26-1.30) phases compared with patients without a documented infection. Specifically, the risk of abdominal pain was higher in COVID-19-positive patients during the postacute (2.54% vs 2.06%; ARR, 1.14; 95% CI, 1.11-1.17) and chronic (4.57% vs 3.40%; ARR, 1.24; 95% CI, 1.22-1.27) phases.
Conclusions and relevance: In this cohort study, the increased risk of GI tract symptoms and disorders was associated with the documented SARS-CoV-2 infection in children or adolescents during the postacute or chronic phase. Clinicians should note that lingering GI tract symptoms may be more common in children after documented SARS-CoV-2 infection than in those without documented infection.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

