E6AP is essential for the proliferation of HPV-positive cancer cells by preventing senescence
- PMID: 39919145
- PMCID: PMC11805377
- DOI: 10.1371/journal.ppat.1012914
E6AP is essential for the proliferation of HPV-positive cancer cells by preventing senescence
Abstract
Oncogenic types of human papillomaviruses (HPVs) are major human carcinogens. The formation of a trimeric complex between the HPV E6 oncoprotein, the cellular ubiquitin ligase E6AP and the p53 tumor suppressor protein leads to proteolytic p53 degradation and plays a central role for HPV-induced cell transformation. We here uncover that E6AP silencing in HPV-positive cancer cells ultimately leads to efficient induction of cellular senescence, revealing that E6AP acts as a potent anti-senescent factor in these cells. Thus, although the downregulation of either E6 or E6AP expression also acts partially pro-apoptotic, HPV-positive cancer cells surviving E6 repression proliferate further, whereas they become irreversibly growth-arrested upon E6AP repression. We moreover show that the senescence induction following E6AP downregulation is mechanistically highly dependent on induction of the p53/p21 axis, other than the known pro-senescent response of HPV-positive cancer cells following combined downregulation of the viral E6 and E7 oncoproteins. Of further note, repression of E6AP allows senescence induction in the presence of the anti-senescent HPV E7 protein. Yet, despite these mechanistic differences, the pathways underlying the pro-senescent effects of E6AP or E6/E7 repression ultimately converge by being both dependent on the cellular pocket proteins pRb and p130. Taken together, our results uncover a hitherto unrecognized and potent anti-senescent function of the E6AP protein in HPV-positive cancer cells, which is essential for their sustained proliferation. Our results further indicate that interfering with E6AP expression or function could result in therapeutically desired effects in HPV-positive cancer cells by efficiently inducing an irreversible growth arrest. Since the critical role of the E6/E6AP/p53 complex for viral transformation is conserved between different oncogenic HPV types, this approach could provide a therapeutic strategy, which is not HPV type-specific.
Copyright: © 2025 Avenhaus et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
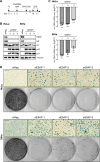
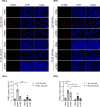





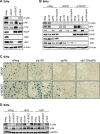
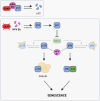
Similar articles
-
Regulation of HPV E7 Stability by E6-Associated Protein (E6AP).J Virol. 2022 Aug 24;96(16):e0066322. doi: 10.1128/jvi.00663-22. Epub 2022 Aug 2. J Virol. 2022. PMID: 35916535 Free PMC article.
-
Regulation of human papillomavirus E6 oncoprotein function via a novel ubiquitin ligase FBXO4.mBio. 2025 Feb 5;16(2):e0278324. doi: 10.1128/mbio.02783-24. Epub 2024 Dec 17. mBio. 2025. PMID: 39688415 Free PMC article.
-
Tanshinone IIA inhibits viral oncogene expression leading to apoptosis and inhibition of cervical cancer.Cancer Lett. 2015 Jan 28;356(2 Pt B):536-46. doi: 10.1016/j.canlet.2014.09.037. Epub 2014 Oct 7. Cancer Lett. 2015. PMID: 25304375
-
Warts, cancer and ubiquitylation: lessons from the papillomaviruses.Trans Am Clin Climatol Assoc. 2006;117:113-26; discussion 126-7. Trans Am Clin Climatol Assoc. 2006. PMID: 18528468 Free PMC article. Review.
-
HPV E6, E6AP and cervical cancer.BMC Biochem. 2008 Oct 21;9 Suppl 1(Suppl 1):S4. doi: 10.1186/1471-2091-9-S1-S4. BMC Biochem. 2008. PMID: 19007434 Free PMC article. Review.
Cited by
-
Pleiotropic Effects of Metformin on the Chemotherapy Response of HPV-Positive Cancer Cells.J Med Virol. 2025 Jun;97(6):e70434. doi: 10.1002/jmv.70434. J Med Virol. 2025. PMID: 40522309 Free PMC article.
-
Hypoxic HPV-Positive Cancer Cells Are Particularly Sensitive to the Pro-Senescent Effects of B-MYB Repression Due to the Lack of Compensatory A-MYB Induction.J Med Virol. 2025 Jun;97(6):e70422. doi: 10.1002/jmv.70422. J Med Virol. 2025. PMID: 40444458 Free PMC article.
References
-
- Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al.. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health. 2023;11(2):e197–e206. doi: 10.1016/S2214-109X(22)00501-0 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

