Cation effects on CO2 reduction catalyzed by single-crystal and polycrystalline gold under well-defined mass transport conditions
- PMID: 39919184
- PMCID: PMC11804923
- DOI: 10.1126/sciadv.adr6465
Cation effects on CO2 reduction catalyzed by single-crystal and polycrystalline gold under well-defined mass transport conditions
Abstract
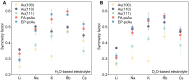
The presence of alkali metal cations in the electrolyte substantially affects the reactivity and selectivity of electrochemical carbon dioxide (CO2) reduction (CO2R). This study examines the role of cations in CO2R on single-crystal and polycrystalline Au under controlled mass-transport conditions. It establishes that CO2 adsorption is the rate-determining step regardless of cation type or surface structure. Density functional theory calculations show that electron transfer occurs to a solvated CO2-cation complex. A more positive potential of zero charge enhances CO2R activity only on Au with similar surface coordination. The symmetry factor (β) of the rate-determining step varies with surface structure and cation identity, with density functional theory calculations indicating β's sensitivity to surface and double-layer structures. These findings emphasize the importance of both surface and double-layer structures in understanding cation effects on CO2R.
Figures






References
-
- Nitopi S., Bertheussen E., Scott S. B., Liu X., Engstfeld A. K., Horch S., Seger B., Stephens I. E. L., Chan K., Hahn C., Nørskov J. K., Jaramillo T. F., Chorkendorff I., Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019). - PubMed
-
- Jones J.-P., Prakash G. K. S., Olah G. A., Electrochemical CO2 reduction: Recent advances and current trends. Isr. J. Chem. 54, 1451–1466 (2014).
-
- Stephens I. E. L., Chan K., Bagger A., Boettcher S. W., Bonin J., Boutin E., Buckley A. K., Buonsanti R., Cave E. R., Chang X., Chee S. W., da Silva A. H. M., de Luna P., Einsle O., Endrődi B., Escudero-Escribano M., de Araujo J. V. F., Figueiredo M. C., Hahn C., Hansen K. U., Haussener S., Hunegnaw S., Huo Z., Hwang Y. J., Janáky C., Jayathilake B. S., Jiao F., Jovanov Z. P., Karimi P., Koper M. T. M., Kuhl K. P., Lee W. H., Liang Z., Liu X., Ma S., Ma M., Oh H.-S., Robert M., Cuenya B. R., Rossmeisl J., Roy C., Ryan M. P., Sargent E. H., Sebastián-Pascual P., Seger B., Steier L., Strasser P., Varela A. S., Vos R. E., Wang X., Xu B., Yadegari H., Zhou Y., 2022 roadmap on low temperature electrochemical CO2 reduction. J. Phys. Energy 4, 042003 (2022).
-
- Akira M., Yoshio H., Product selectivity affected by cationic species in electrochemical reduction of CO2 and CO at a Cu electrode. Bull. Chem. Soc. Jpn. 64, 123–127 (1991).
-
- Ringe S., Clark E. L., Resasco J., Walton A., Seger B., Bell A. T., Chan K., Understanding cation effects in electrochemical CO2 reduction. Energy Environ. Sci. 12, 3001–3014 (2019).
LinkOut - more resources
Full Text Sources

