A SARS-CoV-2 vaccine on an NIR-II/SWIR emitting nanoparticle platform
- PMID: 39919189
- PMCID: PMC11804919
- DOI: 10.1126/sciadv.adp5539
A SARS-CoV-2 vaccine on an NIR-II/SWIR emitting nanoparticle platform
Abstract
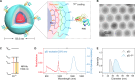
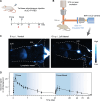
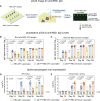
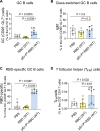
The COVID-19 pandemic caused a global health crisis that resulted in millions of deaths. Effective vaccines have played central roles in curtailing the pandemic. Here, we developed a down-converting near-infrared IIb (NIR-IIb; 1500 to 1700 nanometers) luminescent, pure NaErF4@NaYF4 rare-earth nanoparticle (pEr) as vaccine carriers. The pEr nanoparticles were coated with three layers of cross-linked biocompatible polymers (pEr-P3; ~55 nanometers) and conjugated to the receptor binding domain (RBD) of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein. Upon subcutaneous injection of the pEr-P3-RBD nanovaccine in mice, in vivo NIR-IIb imaging revealed active vaccine trafficking and migration to lymph nodes through lymphatic vessels. Two doses of the adjuvant-free vaccine elicited long-lasting (>7 months) high titers of serum viral neutralization antibody and anti-RBD immunoglobulin G, along with robust RBD-specific germinal center B cells and T follicular helper cells. We devised in vivo NIR-II molecular imaging of RBD-specific cells in lymph nodes, opening noninvasive assessments of vaccine-elicited immune responses longitudinally.
Figures

References
-
- World Health Organization, WHO Coronavirus Disease (COVID-19) Dashboard, https://covid19.who.int/ [accessed 27 June 2023].
-
- World Health Organization, COVID-19 Vaccines with WHO Emergency Use Listing, https://extranet.who.int/prequal/vaccines/covid-19-vaccines-who-emergenc....
-
- World Health Organization, COVID-19 vaccine tracker and landscape, https://who.int/publications/m/item/draft-landscape-of-covid-19-candidat....
-
- Levine-Tiefenbrun M., Yelin I., Alapi H., Katz R., Herzel E., Kuint J., Chodick G., Gazit S., Patalon T., Kishony R., Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat. Med. 27, 2108–2110 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

