An acidic residue within the OCT4 dimerization interface of SOX17 is necessary and sufficient to overcome its pluripotency-inducing activity
- PMID: 39919754
- PMCID: PMC11960519
- DOI: 10.1016/j.stemcr.2025.102398
An acidic residue within the OCT4 dimerization interface of SOX17 is necessary and sufficient to overcome its pluripotency-inducing activity
Abstract
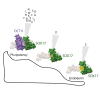
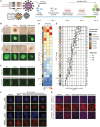
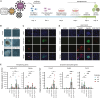
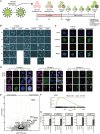
SOX17 directs the differentiation toward endoderm and acts as a human germline specifier. We previously found that the replacement of glutamate at position 57 of the high-mobility group (HMG) box with the basic lysine residue in SOX2 alters interactions with OCT4 and turns SOX17 into a pluripotency factor. Here, we systematically interrogated how mutations at this critical position affect the cellular reprogramming activity of SOX17 in mouse and human. We found that most mutations turn SOX17 into a pluripotency factor regardless of their biophysical properties except for acidic residues and proline. The conservative mutation to an aspartate allows the SOX17E57D protein to maintain a self-renewing endodermal state. We showed that only the glutamate in the wild-type protein blocks the formation of an SOX17/OCT4 dimer at composite DNA elements in pluripotency enhancers. Insights into how modifications of an ultra-conserved residue affect functions of developmental transcription factors provide avenues to advance cell fate engineering.
Keywords: SOX17; SOX2; XEN; engineered proteins; iPSCs; pioneer factors; pluripotency; reprogramming.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests This paper includes results of the doctoral thesis entitled “Systematic analysis of the sequence-function relationship underlying differentiation and dedifferentiation activity of SOX17” submitted by S.Y.H. to the Li Ka Shing Faculty of Medicine, The University of Hong Kong, in 2024.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

