Clinical Assessment of the Drug-Drug Interaction Potential of Omaveloxolone in Healthy Adult Participants
- PMID: 39920097
- PMCID: PMC12110731
- DOI: 10.1002/jcph.6189
Clinical Assessment of the Drug-Drug Interaction Potential of Omaveloxolone in Healthy Adult Participants
Abstract
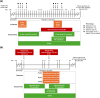
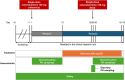
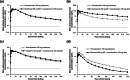
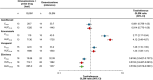
Omaveloxolone is approved in the United States and the European Union for the treatment of patients with Friedreich ataxia aged ≥16 years. It is mainly metabolized by cytochrome P450 (CYP) 3A4 in vitro. Two drug-drug interaction studies (NCT04008186 and NCT05909644) were performed to evaluate (1) the effect of drug-metabolizing enzymes (DMEs) and drug transporter (DT) modulators on the pharmacokinetics of omaveloxolone and (2) the effect of omaveloxolone on the pharmacokinetics of DME and DT substrates. Additionally, the safety of coadministering these drugs with omaveloxolone was assessed. Coadministration of the strong CYP3A4 inhibitor itraconazole significantly increased omaveloxolone maximum plasma concentration (Cmax) and area under the plasma concentration-time curve from time 0 extrapolated to infinity (AUC0-∞) by approximately 3- and 4-fold, respectively. Conversely, coadministration with the moderate CYP3A4 inducer efavirenz decreased Cmax and AUC0-∞ of omaveloxolone by 38.0% and 48.5%, respectively. Omaveloxolone exposure was also increased following coadministration with verapamil, a moderate CYP3A4 and P-glycoprotein (P-gp) inhibitor, but it was unaffected by the strong CYP2C8 inhibitor gemfibrozil. Coadministration of omaveloxolone reduced systemic exposure of the substrates of CYP3A4, CYP2C8, breast cancer resistance protein, and organic anion transporting polypeptide 1B1 but had no effect on those of P-gp and organic cation transporter 1. Omaveloxolone was well tolerated when administered alone and in combination with the DME and DT modulators or substrates. These findings support concomitant medication precautions and dosing recommendations for omaveloxolone when coadministered with a moderate or strong CYP3A4 inhibitor or inducer, as well as the substrates of certain CYP450 enzymes or transporters.
Keywords: CYP3A4; Friedreich ataxia; drug–drug interaction; omaveloxolone; pharmacokinetics.
© 2025 Biogen, Inc. The Journal of Clinical Pharmacology published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
Hamim Zahir, Masako Murai, and Lucy Wu are employees of and may hold stock in Biogen. Scott Hynes was an employee of and may have held stock in Biogen at the time of development of this manuscript. Michelle Valentine is an employee of Celerion, Inc., which was commissioned by Reata Pharmaceuticals to conduct the study; Reata was acquired by Biogen in 2023.
Figures


References
-
- Fichera M, Castaldo A, Mongelli A, et al. Comorbidities in Friedreich ataxia: incidence and manifestations from early to advanced disease stages. Neurol Sci. 2022;43(12):6831‐6838. - PubMed
-
- Parkinson MH, Boesch S, Nachbauer W, Mariotti C, Giunti P. Clinical features of Friedreich's ataxia: classical and atypical phenotypes. J Neurochem. 2013;126(suppl 1):103‐117. - PubMed
-
- Chiang S, Huang MLH, Park KC, Richardson DR. Antioxidant defense mechanisms and its dysfunctional regulation in the mitochondrial disease, Friedreich's ataxia. Free Radic Biol Med. 2020;159:177‐188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

