Mechanistic elucidation of human pancreatic acinar development using single-cell transcriptome analysis on a human iPSC differentiation model
- PMID: 39920294
- PMCID: PMC11806057
- DOI: 10.1038/s41598-025-88690-1
Mechanistic elucidation of human pancreatic acinar development using single-cell transcriptome analysis on a human iPSC differentiation model
Abstract
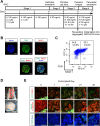
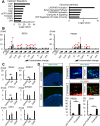
Few effective treatments have been developed for intractable pancreatic exocrine disorders due to the lack of suitable disease models using human cells. Pancreatic acinar cells differentiated from human induced pluripotent stem cells (hiPSCs) have the potential to solve this issue. In this study, we aimed to elucidate the developmental mechanisms of pancreatic exocrine acinar lineages to establish a directed differentiation method for pancreatic acinar cells from hiPSCs. hiPSC-derived pancreatic endoderm cells were spontaneously differentiated into both pancreatic exocrine and endocrine tissues by implantation into the renal subcapsular space of NOD/SCID mice. Single-cell RNA-seq analysis of the retrieved grafts confirmed the differentiation of pancreatic acinar lineage cells and identified REG4 as a candidate marker for pancreatic acinar progenitor cells. Furthermore, differential gene expression analysis revealed upregulated pathways, including cAMP-related signals, involved in the differentiation of hiPSC-derived pancreatic acinar lineage cells in vivo, and we found that a cAMP activator, forskolin, facilitates the differentiation from hiPSC-derived pancreatic endoderm into pancreatic acinar progenitor cells in our in vitro differentiation culture. Therefore, this platform contributes to our understanding of the developmental mechanisms of pancreatic acinar lineage cells and the establishment of differentiation methods for acinar cells from hiPSCs.
Keywords: Acinar cell; Forskolin; Pancreas; REG4; Single-cell transcriptome; iPSC.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: K.O. is a founder and member of the scientific advisory boards of iPS Portal, Inc., and a founder and chief scientific advisor of RegeNephro Co., Ltd. T.T. is a scientific adviser of Orizuru Therapeutics, Inc. A.K. and H.T. are an employee of RegeNephro Co., Ltd. There are no competing interests to declare by the other authors.
Figures


References
-
- Leung, P. S. Overview of the pancreas. Adv. Exp. Med. Biol.690, 3–12. 10.1007/978-90-481-9060-7_1 (2010). - PubMed
-
- Pandol, S. J. The Exocrine Pancreas (Morgan & Claypool Life Sciences, 2010). - PubMed
-
- Steward, M. C., Ishiguro, H. & Case, R. M. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu. Rev. Physiol.67, 377–409. 10.1146/annurev.physiol.67.031103.153247 (2005). - PubMed
-
- In’t Veld, P. & Marichal, M. Microscopic anatomy of the human islet of Langerhans. Adv. Exp. Med. Biol.654, 1–19. 10.1007/978-90-481-3271-3_1 (2010). - PubMed

