Arginine: at the crossroads of nitrogen metabolism
- PMID: 39920310
- PMCID: PMC11876448
- DOI: 10.1038/s44318-025-00379-3
Arginine: at the crossroads of nitrogen metabolism
Abstract
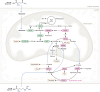
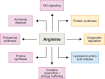
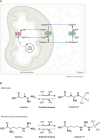
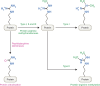
L-arginine is the most nitrogen-rich amino acid, acting as a key precursor for the synthesis of nitrogen-containing metabolites and an essential intermediate in the clearance of excess nitrogen. Arginine's side chain possesses a guanidino group which has unique biochemical properties, and plays a primary role in nitrogen excretion (urea), cellular signaling (nitric oxide) and energy buffering (phosphocreatine). The post-translational modification of protein-incorporated arginine by guanidino-group methylation also contributes to epigenetic gene control. Most human cells do not synthesize sufficient arginine to meet demand and are dependent on exogenous arginine. Thus, dietary arginine plays an important role in maintaining health, particularly upon physiologic stress. How cells adapt to changes in extracellular arginine availability is unclear, mostly because nearly all tissue culture media are supplemented with supraphysiologic levels of arginine. Evidence is emerging that arginine-deficiency can influence disease progression. Here, we review new insights into the importance of arginine as a metabolite, emphasizing the central role of mitochondria in arginine synthesis/catabolism and the recent discovery that arginine can act as a signaling molecule regulating gene expression and organelle dynamics.
Keywords: Arginine Deficiency; Arginine Metabolism; Metabolite Signaling; Mitochondria; Protein Synthesis.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. Craig B Thompson is a founder of Agios Pharmaceuticals. He also serves on the Board of Directors of Regeneron and Charles River Laboratories. The other authors declare no competing interests.
Figures


References
-
- Abou-Alfa GK, Qin S, Ryoo BY, Lu SN, Yen CJ, Feng YH, Lim HY, Izzo F, Colombo M, Sarker D et al (2018) Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol 29:1402–1408 - PubMed
-
- Al-Habsi M, Chamoto K, Matsumoto K, Nomura N, Zhang B, Sugiura Y, Sonomura K, Maharani A, Nakajima Y, Wu Y et al (2022) Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice. Science 378:eabj3510 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

