Can nebulised heparin reduce acute lung injury in patients with SARS‑CoV‑2 requiring advanced respiratory support in Ireland: the CHARTER‑Ireland phase Ib/IIa, randomised, parallel-group, open-label study
- PMID: 39920521
- PMCID: PMC11806160
- DOI: 10.1186/s40635-025-00727-x
Can nebulised heparin reduce acute lung injury in patients with SARS‑CoV‑2 requiring advanced respiratory support in Ireland: the CHARTER‑Ireland phase Ib/IIa, randomised, parallel-group, open-label study
Abstract
Background: Nebulised unfractionated heparin may attenuate COVID-19 ARDS by reducing pulmonary microvascular thrombosis, blocking SARS-CoV-2 entry into cells, and decreasing lung inflammation. COVID-19 patients with a raised D-dimer have areas of pulmonary hypoperfusion on CT perfusion scans of the lung and have increased mortality risk.
Methods: This was a phase Ib/IIa open-label multi-centre, randomised controlled trial. The study was designed to evaluate whether nebulised unfractionated heparin decreased D-dimer concentrations, with safety as a co-primary outcome.
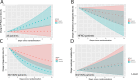
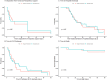
Results: Forty patients were recruited, with 20 patients into each group. Mean age was 56.6 (SD 11.5) in the heparin group and 51.3 (SD 14.7) in the standard care group, while 60% of participants were male. There was no change in D-dimers from baseline to day 10 (heparin group mean change - 316.5, [SD 1840.3] and control group mean change - 321.7 [SD 3589.4]; p = 0.996). Fourteen patients suffered at least one serious adverse event, 9 patients the Heparin group and 5 in the control group. Eight patients had one or more bleeding events, 5 in the heparin group and 3 in the control group, but were no cases of pulmonary bleeding, of severe haemorrhage or of heparin-induced thrombocytopenia. Patients receiving heparin therapy had lower PaO2/FiO2 ratios, increased oxygenation indices, and decreased ROX index profiles, up to day 10. The time to separation from respiratory support, and the time to ICU or hospital discharge was similar in both groups. There were 3 deaths in the Heparin group and 2 in the control group.
Conclusions: Nebulised unfractionated heparin was safe and well tolerated, but did not reduce D-dimer concentrations, and worsened oxygenation indices in patients with COVID-19 ARDS.
Keywords: Acute respiratory distress syndrome; Aerosol delivery; COVID-19; Heparin; Nebulised; Safety study.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethics approval was obtained from the National Research Ethics Committee (NREC) in Ireland, approval number 20-NREC-COV-104. Regulatory approval for the study was obtained from the Health Products Regulatory Authority, approval number CT0900/650/001 Heparin Sodium. Written informed consent or assent was obtained from all patients prior to inclusion in the trial for collection, storage, analysis and dissemination of the results of the trial. The trial was conducted in accordance with the Declaration of Helsinki. Consent for publication: Written informed consent or assent was obtained from all patients prior to inclusion in the trial for publication and dissemination of the results of the trial. Availability of data and materials: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. Competing interests: JL is a senior editor of Intensive Care Medicine Experimental. JL has received consulting fees from Cellenkos Inc. All other authors declare that they have no competing interests.
Figures


References
-
- Dixon B, Smith RJ, Campbell DJ, Moran JL, Doig GS, Rechnitzer T, MacIsaac CM, Simpson N, van Haren FMP, Ghosh AN, Gupta S, Broadfield EJC, Crozier TME, French C, Santamaria JD, CHARLI Study Group (2021) Nebulised heparin for patients with or at risk of acute respiratory distress syndrome: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med 9:360–372 - PMC - PubMed
-
- Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, Laffey J, Carrafiello G, Carsana L, Rizzuto C, Zanella A, Scaravilli V, Pizzilli G, Grieco DL, Di Meglio L, de Pascale G, Lanza E, Monteduro F, Zompatori M, Filippini C, Locatelli F, Cecconi M, Fumagalli R, Nava S, Vincent JL, Antonelli M, Slutsky AS, Pesenti A, Ranieri VM, Collaborators (2020) Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med 8:1201–1208 - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

