Evaluation of three novel antigens and costimulatory agents for improvement of M. Tuberculosis specific interferon gamma release assays
- PMID: 39920589
- PMCID: PMC11806546
- DOI: 10.1186/s12879-025-10577-3
Evaluation of three novel antigens and costimulatory agents for improvement of M. Tuberculosis specific interferon gamma release assays
Abstract
Background: Mycobacterium tuberculosis (MT) infections represent a global health problem and latent tuberculosis infection (LTBI) affects an estimated 25% of the world population. 10.6 million people fell ill with tuberculosis (TB) worldwide in 2021 and a total of 1.6 million TB-associated deaths were reported. Thus, reliable diagnosis of LTBI is crucial to ensure adequate treatment. We tested three novel MT antigens of the dormancy survival regulator (DosR) complex, ACR, Rv1733, Rv2626, for improvement of MT specific interferon gamma release assays (IGRA) for diagnosing TB. Furthermore, we specifically investigated the potential of the complement factor C5a and the toll like receptor (TLR) agonists CpG ODN as well as Poly(I: C) as costimulators in order to increase diagnostic quality of MT IGRAs. Three MT IGRAs were evaluated, i.e. our in-house IGRA, a prototypic EUROIMMUN Quan-T-Cell TB assay and the gold standard QuantiFERON Tb-Gold Plus assay.
Methods: In this single-center, prospective trial, whole blood from 71 patients with tuberculosis disease was stimulated using our in-house IGRA with ACR, Rv1733, Rv2626 compared to the current gold standard MT antigen formulation encompassing MT antigens ESAT-6, CFP-10 and TB10.4. Further, C5a, CpG ODN and Poly(I: C) were tested as co-stimulators. IFN-γ levels in plasma were quantified using ELISA.
Results: The three novel antigens ACR, Rv1733 and Rv2626 failed to elicit equal or stronger IFN-γ-responses compared to the gold standard antigen formulation with ESAT-6, CFP-10 and TB10.4. The TLR9 agonist CpG ODN increased IFN-γ responses in whole blood of tuberculosis patients using our in-house assays (6,768 ± 21,097 mlU/ml vs. 2,971 ± 4,780 mlU/ml, p = 0.31), yet not significantly. The same trend was found for the prototypic EUROIMMUN Quan-T-Cell TB assay (3,355 ± 5,425 mlU/ml vs. 2,548 ± 4,145 mlU/ml, p = 0.1) and the QuantiFERON Tb-Gold Plus assay (3,627 ± 5,992 mlU/ml vs. 2,635 m ± 4,475 mlU/ml, p = 0.08, for tube 1; 3,257 ± 5,349 vs. 2,759 ± 4,446 mIU/ml, p = 0.25, for tube 2). No increase of IFN-γ release was seen using Poly(I: C) or C5a in all three assays.
Conclusions: ACR, Rv1733 and Rv2626 failed to elicit equal or even better IFN-γ responses in our in-house IGRA compared to ESAT-6, CFP-10 and TB10.4 in patients with MT infection. The TLR9 agonist CpG ODN might be useful as co-stimulator in MT IGRAs.
Keywords: Complement factors; IGRA; Interferon gamma release assay; TLR agonists; Tuberculosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Our study adhered to the Declaration of Helsinki regarding research carried out on humans and/or human data. The study was approved by the Ethics Committee of the Brandenburg Chamber of Physicians (S20(a)/2016) as well as the Berlin Chamber of Physicians (Eth-V-ZK/17) and all patients gave informed consent for the participation. Consent for publication: Not applicable. Competing interests: D.Z. and V.H. are employed by EUROIMMUN, a manufacturer of diagnostic reagents and co-owner of patents related to serological assays for the diagnosis of infectious diseases and the detection of immunity as a result of vaccination. EUROIMMUN provided kits for analyses at discounted rates. The other authors declare that they have no competing interests.
Figures
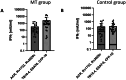

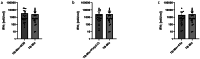

References
-
- World Health O. Global tuberculosis report 2021. Geneva: World Health Organization; 2021 2021.
-
- Long R, Divangahi M, Schwartzman K. Chapter 2: transmission and pathogenesis of tuberculosis. Can J Respiratory Crit Care Sleep Med. 2022;6(sup1):22–32.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

