Liberal versus restrictive transfusion strategies in subarachnoid hemorrhage: a secondary analysis of the TRAIN study
- PMID: 39920710
- PMCID: PMC11803982
- DOI: 10.1186/s13054-025-05270-5
Liberal versus restrictive transfusion strategies in subarachnoid hemorrhage: a secondary analysis of the TRAIN study
Abstract
Background: The optimal hemoglobin (Hb) threshold to trigger red blood cell transfusions (RBCT) in subarachnoid hemorrhage (SAH) patients is unclear. This study evaluated the impact of liberal versus restrictive transfusion strategies on neurological outcome in patients with SAH.
Methods: This is a pre-planned secondary analysis of the "TRansfusion Strategies in Acute brain INjured Patients" (TRAIN) study. We included all SAH patients from the original study that were randomized to receive RBCT when Hb levels dropped below 9 g/dL (liberal group) or 7 g/dL (restrictive group). The primary outcome was an unfavorable neurological outcome at 180 days, defined by a Glasgow Outcome Scale Extended score of 1-5.
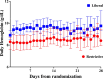
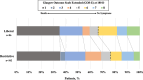
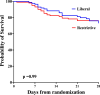
Results: Of the 190 SAH patients in the trial, 188 (98.9%) had data available for the primary outcome, with 86 (45.3%) in the liberal group and 102 (53.6%) in the restrictive group. Patients in the liberal group were older than in the restrictive group, but otherwise had similar baseline characteristics. Patients in the liberal group received more RBCT and showed higher Hb levels over time. At 180 days, 57 (66.3%) patients in the liberal group and 78 (76.4%) in the restrictive group had unfavorable outcomes (risk ratio, RR 0.87; 95% confidence intervals, 95% CI 0.71-1.04). Patients in the liberal group had a significantly lower risk of cerebral ischemia (RR 0.63; 95% CI 0.41-0.97). In a multivariate analysis, randomization to the liberal group was associated with a lower risk of unfavorable outcome (RR 0.83, 95% CI 0.70-0.99).
Conclusions: A liberal transfusion strategy was not associated with a lower incidence of unfavorable outcome after SAH when compared to a restrictive strategy. However, in a multivariable analysis adjusted for confounders randomization to the liberal group was associated with lower risk of unfavorable outcome. The occurrence of cerebral ischemia was significantly lower in the liberal transfusion strategy group.
Trial registration: ClinicalTrials.gov number-NCT02968654 registered on November 16th, 2016.
Keywords: Acute brain injury; Anemia; Blood; Stroke.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This is a secondary analysis of the TRAIN study. The TRAIN study was approved by the ethics committees in each hospital. The primary ethics committee, “Comite d’Ethique Erasme-ULB”, approved this multicentric study on the 14th of March 2016 (P2015/327). Written informed consent was obtained from a legal surrogate before enrollment. Whenever possible, deferred consent was also obtained from the patients who regained mental capacity. Consent for publication: Not applicable. Competing interests: Jean Louis Vincent is editor in chief of critical care. No other authors have reported competing interests.
Figures
References
-
- Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389(10069):655–66. - PubMed
-
- Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D, Investigators ABC. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499–507. - PubMed
-
- Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS, Shapiro MJ. The CRIT Study: Anemia and blood transfusion in the critically ill–current clinical practice in the United States. Crit Care Med. 2004;32(1):39–52. - PubMed
-
- Kramer AH, Zygun DA, Bleck TP, Dumont AS, Kassell NF, Nathan B. Relationship between hemoglobin concentrations and outcomes across subgroups of patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2009;10(2):157–65. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

