Paracrine activity of Smurf1-silenced mesenchymal stem cells enhances bone regeneration and reduces bone loss in postmenopausal osteoporosis
- PMID: 39920824
- PMCID: PMC11806587
- DOI: 10.1186/s13287-025-04165-0
Paracrine activity of Smurf1-silenced mesenchymal stem cells enhances bone regeneration and reduces bone loss in postmenopausal osteoporosis
Abstract
Background: Osteoporosis (OP), characterized by reduced bone mass and mineral density, is a global metabolic disorder that severely impacts the quality of life in affected individuals. Although current pharmacological treatments are effective, their long-term use is often associated with adverse effects, highlighting the need for safer, more sustainable therapeutic strategies. This study investigates the pro-osteogenic and anti-resorptive potential of the secretome from Smurf1-silenced mesenchymal stem cells (MSCs) as a promising cell-free therapy for bone regeneration.
Methods: Conditioned media (CM) from Smurf1-silenced rat (rCM-Smur1) and human MSCs (hCM-Smurf1) was collected and analyzed. Pro-osteogenic potential was assessed by measuring in vitro mineralization in human and rat MSCs cultures. In vivo, studies were conducted using a rat ectopic bone formation model and a post-menopausal osteoporotic mouse model. Additionally, primary human osteoporotic MSCs were preconditioned with hCM-Smurf1, and their osteogenic capacity was compared to that induced by BMP2 treatment. Ex vivo, human bone explants were treated with hCM-Smurf1 to assess anti-resorptive effects. Proteomic analysis of the soluble and vesicular CM fractions identified key proteins involved in bone regeneration.
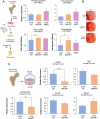
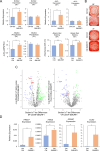
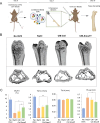
Results: CM from Smurf1-silenced MSCs significantly enhanced mineralization in vitro and bone formation in vivo. Preconditioning human osteoporotic MSCs with hCM-Smurf1 significantly increases in vitro mineralization, with levels comparable to those achieved with BMP2 treatment. Additionally, in ex vivo human bone cultures, treatment with hCM-Smurf1 significantly reduced RANKL expression without affecting OPG levels, indicating an anti-resorptive effect. In vivo, CM from Smurf1-silenced MSCs significantly increased bone formation in a rat ectopic model, and its local administration reduced trabecular bone loss by 50% in a post-menopausal osteoporotic mouse model after a single administration within just four weeks. Proteomic analysis revealed both soluble and vesicular fractions of hCM-Smurf1 were enriched with proteins essential for ossification and extracellular matrix organization, enhancing osteogenic differentiation.
Conclusions: The Smurf1-silenced MSCs' secretome shows potent osteogenic and anti-resorptive effects, significantly enhancing bone formation and reducing bone loss. This study provides compelling evidence for the therapeutic potential of Smurf1-silenced MSC-derived secretome as a non-toxic and targeted treatment for osteoporosis. These findings warrant further in vivo studies and clinical trials to validate its therapeutic efficacy and safety.
Keywords: Smurf1; Mesenchymal stem cells; Osteoporosis; Secretome.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted according to the guidelines of the Declaration of Helsinki. This study was approved by the Institutional Bioethics Committee of the University of Cantabria (Reference 2015/21; Project Title: Enhancing Mesenchymal Stem Cells Osteogenic Capacity Through Modulation of the Bone Marrow Microenvironment; date of approval 04–11-22) and the Consejería de Agricultura y Ganadería de Cantabria (Reference PI-08–21; Project Title: Modulation of the Regenerative Capacity of Mesenchymal Stem Cells for Their Use in Therapies Applied to the Musculoskeletal System; date of approval 01–11-21). Bone samples were obtained from the patients after anonymization. Informed consent was obtained from all the subjects involved in the study. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures



References
-
- Khosla S, Riggs BL. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin North Am. 2005;34(4):1015–30. - PubMed
-
- Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86(3–5):225–30. - PubMed
-
- Riggs BL, Melton LJ 3rd. The prevention and treatment of osteoporosis. N Engl J Med. 1992;327(9):620–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

