Exploring the role of ESR1 mutations in metastatic hormone receptor-positive breast cancer T cell immune surveillance disruption
- PMID: 39920833
- PMCID: PMC11806781
- DOI: 10.1186/s13058-025-01962-6
Exploring the role of ESR1 mutations in metastatic hormone receptor-positive breast cancer T cell immune surveillance disruption
Abstract
Background: Breast Cancer (BC) is the most common type of cancer in women around the world and 70% of cases are hormone-receptor positive (HR+). In 40% of cases, a key mechanism of endocrine resistance to the standard first line is a mutation of the ligand-binding domain (LBD) of Estrogen Receptor 1 (ESR1) encoding estrogen receptor α (ER). Most common ESR1 mutations that occur at positions 537 and 538 have been associated with poor clinical outcomes. ESR1 mutations have the potential to provide neoantigens. This study aims to identify if ESR1 mutations generate specific T cell responses against ESR1 neoantigens in patients with HR+ HER2- BC, and to investigate if ESR1 mutations might correlate with a gene expression profile related to immune surveillance disruption.
Methods: We identified candidate ESR1-derived peptides by predictive software (SYFPEITHI and NetMHCpan 3.0). Then the immunogenicity of ESR1-derived peptides was assessed in Peripheral-Blood-Mononuclear-Cells from 31 healthy donors (HD) and 25 patients with metastatic HR-positive BC by IFN-γ ELISpot assay. A vaccination assay on a humanized mouse model (HLA-A2/DR1) was used to validate the immunogenicity and the presentation of these peptides. Finally, we used Bulk RNA-Seq sequencing along with MCPcounter, a cellular deconvolution method, to investigate the immune contexture of ESR1-mutated BC.
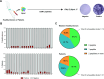
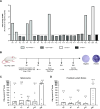
Results: Preliminary results showed recognition of ESR1-derived peptides by women HD lymphocytes but not in men. Frequencies and intensities of such immune responses were increased in patients with BC. Our results showed that 40% of patients had specific immune responses. In addition, we demonstrated the HLA-A2 ESR1 peptide immunogenicity in humanized HLA-A2/DR1 mice. In a data set generated from BC patients refractory to conventional therapy we showed that ESR1 mutations are correlated in advanced diseases with downregulation of molecules involved in antigen presentation and with loss HLA Class I gene expression. ESR1-mutated BC had a decrease in immune cell infiltration.
Conclusion: These results support that common ESR1 mutations generate neoantigens in hormone-receptor positive metastatic breast cancers. If ESR1 peptides-restricted lymphocytes were detectable in BC patients, ESR1 mutations promote immune escape at advanced stages.
Trial registration: ClinicalTrials.gov, NCT02838381. Registered on June 2012.
Keywords: ESR1 mutation; Hormone-receptor positive breast cancer; Neoantigen; Specific immune responses; T cell.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the ethical standards set forth by the CRC01 protocol, cohort D, and was approved under NCT02838381 number. All participants provided written informed consent prior to their inclusion in the study. The consent process included a detailed explanation of the study. Participants were assured that their participation was voluntary and that they could withdraw from the study at any time without any consequence to their medicam care or legal rights. The confidentiality of the participants’ information was strictly maintained throughout the study. The datasets used in this manuscript are available upon reasonable request (METAPRISM (PMID: 36862804) EGAD00001009684). For more detailed information, see the methods section, Bioinformatics procedure/Analysis of database part. Consent for publication: All authors have given consent for publication. Competing interests: The authors declare no competing interests.
Figures


References
-
- Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol off J Am Soc Clin Oncol oct. 1984;2(10):1102–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

