Harnessing tomato-derived small extracellular vesicles as drug delivery system for cancer therapy
- PMID: 39920889
- PMCID: PMC11812386
- DOI: 10.1080/20565623.2025.2461956
Harnessing tomato-derived small extracellular vesicles as drug delivery system for cancer therapy
Abstract
Aim: This study aims to explore a sustainable and scalable approach using tomato fruit-derived sEVs (TsEVs) to deliver calcitriol for enhanced anticancer effects, addressing challenges of low yield and high costs associated with mammalian cell-derived sEVs.
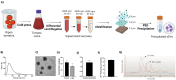
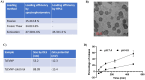
Methods: TsEVs were isolated by centrifugation and ultrafiltration and characterized using DLS, TEM, and biochemical assays. Calcitriol was loaded into TsEVs via loading methods, with efficiency measured by spectrophotometry and HPLC. HCT116 and HT29 colon cancer cells were treated with TsEV-calcitriol and assessed for viability, colony formation, migration, ROS levels, and apoptosis gene expression.
Results: Isolated TsEVs ranged from 30-200 nm with a protein-to-lipid ratio of ∼1. Calcitriol encapsulation efficiencies were 15.4% (passive), 34.8% (freeze-thaw), and 47.3% (sonication). TsEV-calcitriol reduced HCT116 cell viability with IC50 values of 4.05 µg/ml (24 h) and 2.07 µg/ml (48 h). Clonogenic assays showed reduced colony formation and migration. Elevated ROS levels and increased Bax/Bcl-2 ratio were observed in treated HCT116 and HT29 colon cancer cells.
Conclusion: These findings highlight TsEVs as a promising alternative drug delivery platform to mammalian cell-derived sEV for enhancing the therapeutic efficiency of calcitriol and other anticancer agents.
Keywords: Plant exosomes; calcitriol; cancer therapy; cell death; drug delivery; vitamin D.
Plain language summary
This study explores using tiny particles from tomatoes, known as small extracellular vesicles (TsEVs), to deliver calcitriol, a type of vitamin D, as a treatment for colon cancer. These tomato-derived particles offer a cost-effective and sustainable alternative to drug carriers traditionally made from animal cells. TsEVs were successfully loaded with calcitriol, which effectively reduced the growth, spread, and survival of colon cancer cells in lab tests. Additionally, treated cancer cells showed increased levels of stress markers and signals leading to cell death. This approach demonstrates a promising, natural way to improve cancer treatment while addressing cost and supply challenges.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures




References
-
- Kumar SK, Sasidhar MV.. From fluids to forecasts: the promise of small extracellular vesicle mirnas in revolutionising cancer diagnostics. IntechOpen. 2024. doi: 10.5772/intechopen.1005059 - DOI
-
- Kar R, Dhar R, Mukherjee S, et al. . Exosome-based smart drug delivery tool for cancer theranostics. ACS Biomater Sci Eng. 2023;9(2):577–594. doi: 10.1021/acsbiomaterials.2c01329 - DOI - PMC - PubMed
-
•• Highlight the emerging potential of plant-derived sEVs as versatile tool for biomedical applications
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
