Folic acid supplementation in children with sickle cell disease: a randomized double-blind noninferiority cross-over trial
- PMID: 39921095
- PMCID: PMC12002192
- DOI: 10.1016/j.ajcnut.2025.02.001
Folic acid supplementation in children with sickle cell disease: a randomized double-blind noninferiority cross-over trial
Abstract
Background: Children with sickle cell disease (SCD) in Canada are routinely supplemented with folic acid to provide sufficient folate for the increased demands of erythropoiesis. However, with the mandatory folic acid fortification of refined grains and pharmacotherapies that extend the lifespan of sickled red blood cells (RBC), this clinical practice is in question.
Objectives: This study aims to determine the efficacy of folic acid supplementation by measuring the effect of 12 ± 1 wk of 1 mg/d folic acid, compared with placebo, on concentrations of RBC folate (primary outcome), serum folate, and 1-carbon-related metabolites, and clinical outcomes in children with SCD.
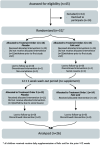
Methods: In this double-blind randomized controlled noninferiority cross-over trial, 31 children with SCD, aged 2-19 y, were enrolled and randomly assigned (1:1 with blocks of 4) to 1 mg/d folic acid, the current standard of care, or a placebo for 12 ± 1 wk. After a 12 ± 1 wk washout period, treatments were reversed.
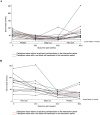
Results: The mean [95% confidence interval (CI)] difference in endline RBC folate concentrations across treatments was -179 (-260, -99) nmol/L, with the lower boundary of the CI exceeding noninferiority but the upper boundary not (P = 0.0001; modified intention-to-treat). There was no significant difference in the number of participants who had RBC folate deficiency after each treatment (P = 0.059). No participants presented with serum folate deficiency (<7 nmol/L). There were no significant differences observed in 1-carbon metabolite concentrations (total homocysteine, S-adenosylhomocysteine, S-adenosylmethionine, vitamin B12, or methylmalonic acid), hematological measures, nor clinical outcomes (specifically acute pain episodes or megaloblastic changes) when individuals were supplemented with folic acid in comparison with placebo.
Conclusions: Despite mandatory food fortification and advances in the medical treatment of SCD, it appears that some children with this condition may still benefit from daily folic acid supplementation. Whether this translates to improved clinical outcomes remains uncertain. This trial was registered at clinicaltrials.gov as NCT04011345 (https://clinicaltrials.gov/study/NCT04011345).
Keywords: folic acid; micronutrient supplementation; one carbon metabolism; pediatrics; randomized control trial; sickle cell disease.
Copyright © 2025 American Society for Nutrition. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors report no conflicts of interest.
Figures


References
-
- Schnog J.B., Duits A.J., Muskiet F.A., ten Cate H., Rojer R.A., Brandjes D.P. Sickle cell disease: a general overview, Neth. J. Med. 2004;62(10):364–374. - PubMed
-
- Koury M.J., Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu. Rev. Nutr. 2004;24:105–131. - PubMed
-
- The Canadian Haemoglobinopathy Association . 2015. Consensus statement on the care of patients with sickle cell disease in Canada. Internet. [cited 27 May 2024]
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

