A Randomized Hybrid-Effectiveness Trial Comparing Pharmacogenomics (PGx) to Standard Care: The PGx Applied to Chronic Pain Treatment in Primary Care (PGx-ACT) Trial
- PMID: 39921243
- PMCID: PMC11805805
- DOI: 10.1111/cts.70154
A Randomized Hybrid-Effectiveness Trial Comparing Pharmacogenomics (PGx) to Standard Care: The PGx Applied to Chronic Pain Treatment in Primary Care (PGx-ACT) Trial
Abstract
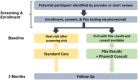
This trial aimed to identify the effects of providing pharmacogenomic (PGx) results and recommendations for patients with chronic pain treated in primary care practices compared to standard care. An open-label, prospective, largely virtual, type-2 hybrid effectiveness trial randomized participants to PGx or standard care arms. Adults with pain ≥ 3 months who were treated with tramadol, codeine, or hydrocodone enrolled. Alternative analgesics were recommended for CYP2D6 intermediate or poor metabolizers (IM/PMs). Prescribing decisions were at providers' discretion. The trial randomized 253 participants. A modified intent-to-treat primary analysis assessed change in pain intensity over 3 months among IM/PMs (PGx: 49; Standard care: 57). The PGx and standard care arms showed no difference in pain intensity change (-0.10 ± 0.63 vs. -0.21 ± 0.75 standard deviation; p = 0.74) or PGx-aligned care (69% vs. 63%; standardized difference [SD] = 0.13). In IM/PMs, secondary analyses of pain intensity change suggested improvements with PGx-aligned (n = 70; -0.21 ± 0.70) vs. unaligned care (n = 36; -0.06 ± 0.69) (SD = -0.22), with this difference increasing when examining IM/PMs with an analgesic change (aligned: n = 31, -0.28 ± 0.76; unaligned: n = 36, -0.06 ± 0.69; SD = -0.31). This approach to PGx implementation for chronic pain was not associated with different prescribing (i.e., similar proportions of PGx-aligned care) or clinical outcomes. Secondary analyses suggest that prescribing aligned with PGx recommendations showed a small improvement in pain intensity. However, the proportion of patients with a clinically meaningful improvement (≥ 30%) in pain intensity was similar. Future efforts should identify effective implementation methods.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
DMS reports research funding to the institution from Kailos Genetics Inc. SMS reports personal Fees for consulting/advisory services/nonpromotional speaking: AstraZeneca, Daiichi‐Sankyo, Genentech/Roche, Sanofi, Merck, Lilly, Chugai Pharmaceutical Co.; Research support (to institution) Genentech/Roche, Kailos Genetics Inc.; Scientific Advisory Board: Napo Pharmaceuticals; Board of Directors: SEAGEN Stock and Stock options (end 12/14/2023), Immunome; Stipend and stock options: Immunome; Other support: Genentech/Roche and AstraZeneca (third‐party writing assistance); In Kind Travel: Seagen, Napo Pharmaceuticals, Sanofi, Daiichi Sankyo, Genentech/Roche, Chugai Pharmaceutical Co. All other authors report no conflicts of interest.
Figures
References
-
- US Department of Health and Human Services , Pain Management Best Practices Inter‐Agency Task Force Report: Updates, Gaps, Inconsistencies, and Recommendations (US Department of Health and Human Services, 2019).
-
- Medical Expenditure Panel Survey (MEPS) , Agency for Healthcare Research and Quality (AHRQ) (ClinCalc DrugStats Database version 2024.01, 2013. –2021), https://clincalc.com/DrugStats/Top300Drugs.aspx.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical